Figure 3:
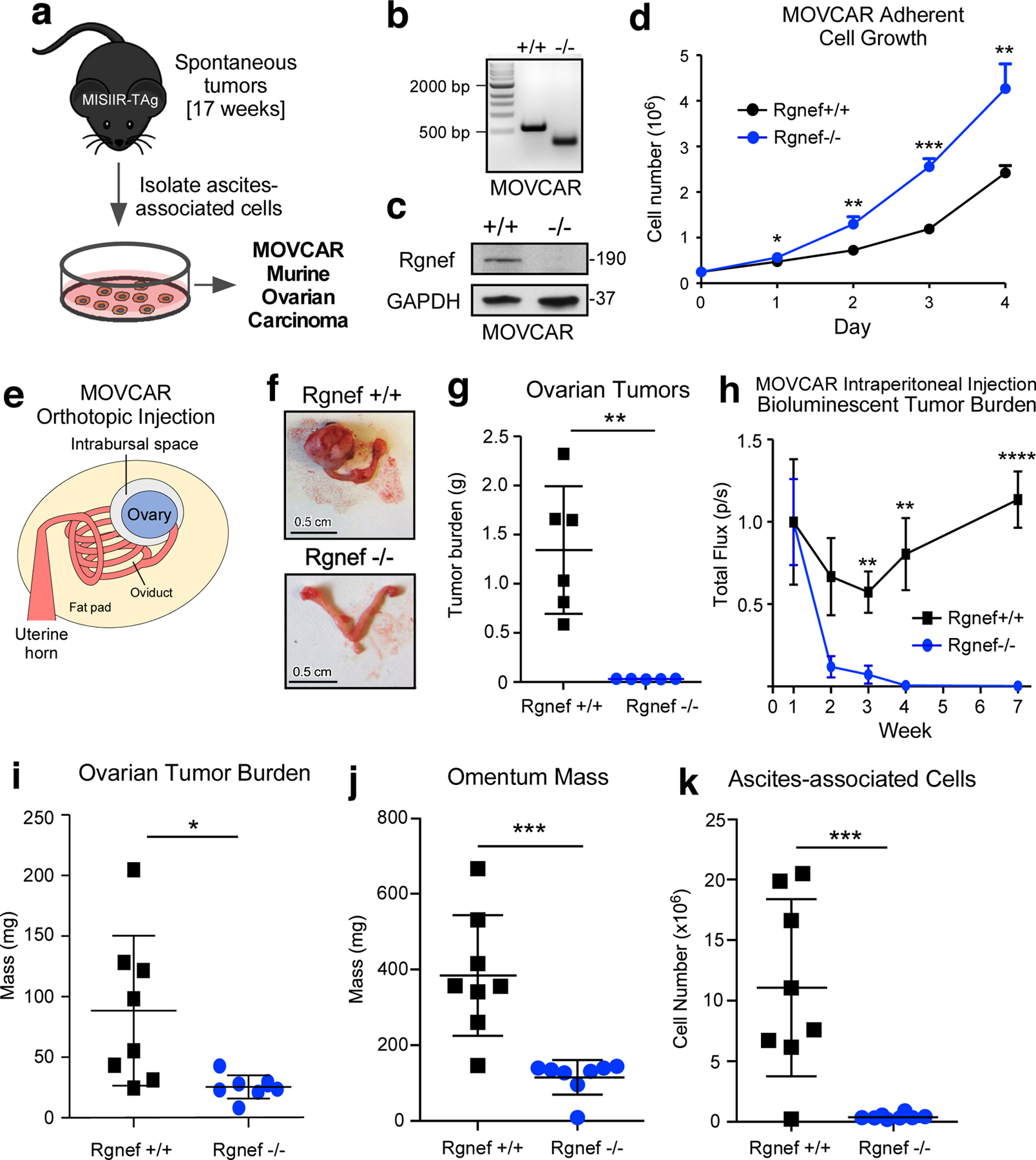
Cell-intrinsic role for Rgnef in promoting murine ovarian cancer (MOVCAR) tumor growth. (a) Schematic of Rgnef+/+ and Rgnef−/− MOVCAR generation. (b) Representative Rgnef exon 24 PCR of MOVCAR cells from Rgnef+/+ and Rgnef−/− TAg+ mice confirming genomic wildtype Rgnef (582 bp) or deletion in Rgnef murine exon 24 (125 bp). (c) Immunoblotting showing Rgnef loss in Rgnef−/− MOVCARs using GAPDH as loading control. (d) Rgnef−/− MOVCARs grow faster in adherent conditions over 4 days (* P ≤ 0.05, ** P ≤ 0.01, ***P ≤0.001, +/−SD, n=3 independent experiments). (e) Schematic of MOVCAR cell orthotopic intrabursal injection into syngeneic MISIIR-TAg-Low; Rgnef+/+ mice. (f) Representative images of oviducts and ovaries from Rgnef+/+ and Rgnef−/− MOVCAR tumor-bearing mice. Scale is 0.5 cm. (g) Quantitation of Rgnef+/+ and Rgnef−/− MOVCAR orthotopic tumor growth (**P ≤ 0.01, +/− SD). (h) Bioluminescent imaging from mice injected intraperitoneally with Rgnef+/+ or Rgnef−/− MOVCARs. Total flux (photons/second) levels are normalized by group to week 1 for each cohort (**P ≤ 0.01, ****P ≤ 0.0001, n=8 for each group, +/− SEM). (i-k) Following intraperitoneal injection, ascites-associated cells were collected at week 14. Rgnef loss impaired metastatic potential as measured by (i) ovarian tumor burden, (j) omentum mass and (k) number of ascites-associated cells (*P ≤ 0.05, ***P ≤ 0.001, +/− SD, n=8 per group).
