Figure 7:
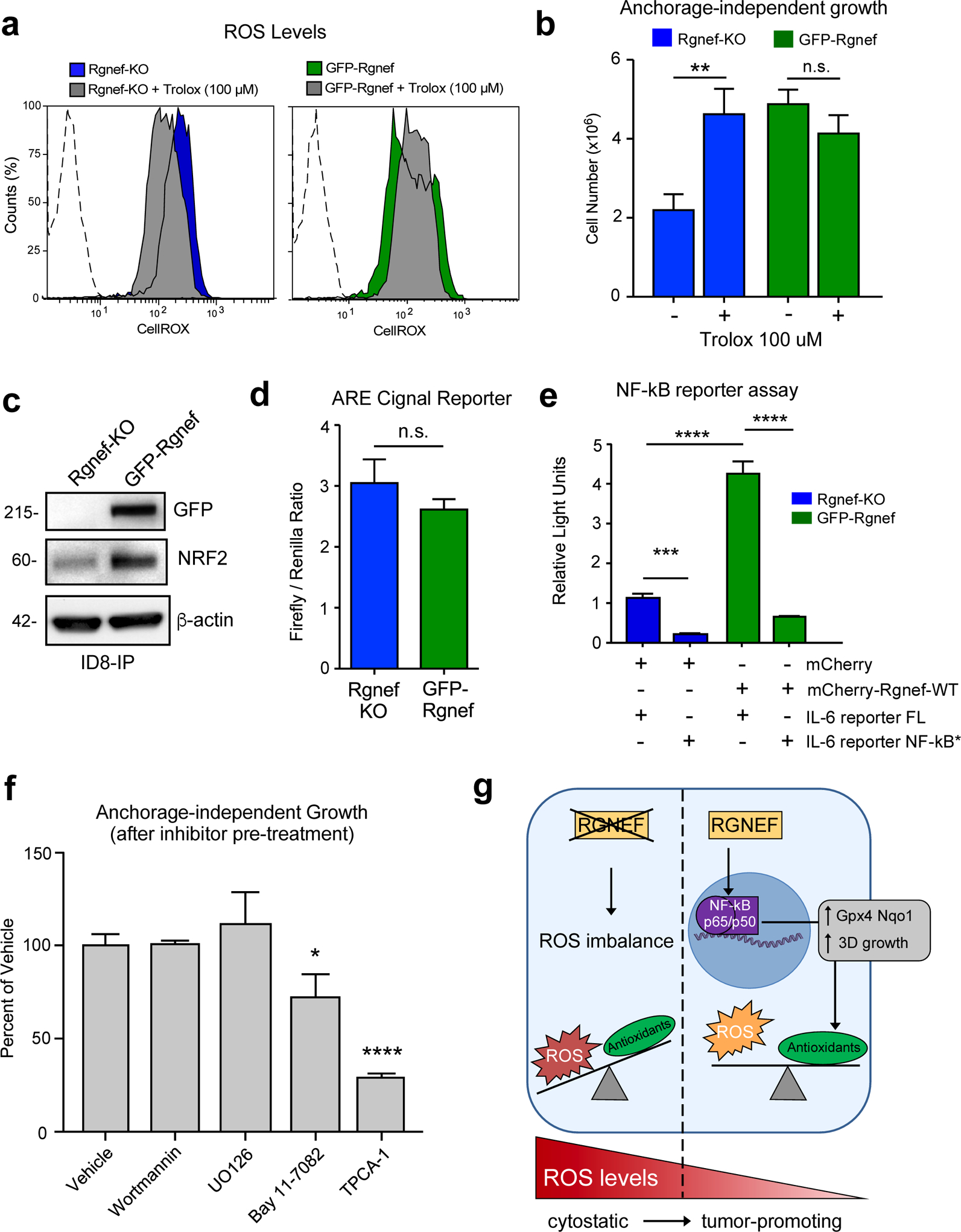
Rgnef supports NF-κB activation needed for ID8-IP anchorage-independent cell growth. (a) Cellular reactive oxygen species (ROS) levels in ID8-IP Rgnef-KO and GFP-Rgnef re-expressing cells as measured by CellROX staining with or without antioxidant Trolox (100 μM, 1 h) addition. Flow cytometry histograms are shown. Negative control (dotted line). (b) Trolox increases ID8-IP Rgnef-KO growth in suspension (** P ≤ 0.01, n=3 independent experiments, +/− SD). (c) Immunoblot of ID8-IP Rgnef KO and GFP-Rgnef cell lysates for GFP, NRF2, and actin protein levels. (d) GFP-Rgnef expression did not enhance ARE (antioxidant response element) reporter for NRF2 transcriptional activity in human 293T cells (n.s.= no significance, +/− SD, n=3 technical replicates). (e) mCherry-Rgnef transfection activates an IL-6 luciferase reporter in human 293T cells dependent on NF-κB DNA binding site integrity (NF-κB*). Dual-luciferase activity was measured and normalized (***P ≤ 0.001, ****P ≤ 0.0001, +/− SD, n=3 technical replicates). (f) Adherent ID8-IP GFP-Rgnef cells were pre-treated with DMSO (vehicle), 1 μM wortmannin, 10 μM U0126, 10 μM Bay 11–7082, or 10 μM TPCA-1 for 24h, washed, and then evaluated for anchorage-independent growth (n.s.=no significance, *P ≤ 0.05, ****P ≤ 0.0001, +/− SD, n=3 technical replicates). (g) Model of Rgnef in mediating cell response to oxidative stress. Upon Rgnef loss, the cell is not able to balance increased ROS resulting from growth in suspension, resulting in oxidative stress. When Rgnef is re-expressed, NF-κB-mediated transcription downstream of Rgnef promotes the expression of an antioxidant gene signature, resulting in redox homeostasis.
