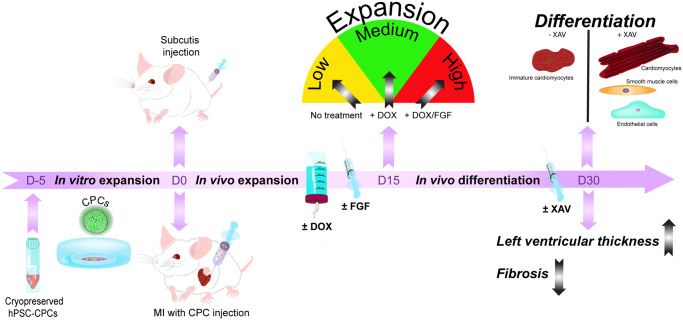
Abstract
Aims
Cardiovascular diseases caused by loss of functional cardiomyocytes (CMs) are a major cause of mortality and morbidity worldwide due in part to the low regenerative capacity of the adult human heart. Human pluripotent stem cell (hPSC)-derived cardiovascular progenitor cells (CPCs) are a potential cell source for cardiac repair. The aim of this study was to examine the impact of extensive remuscularization and coincident revascularization on cardiac remodelling and function in a mouse model of myocardial infarction (MI) by transplanting doxycycline (DOX)-inducible (Tet-On-MYC) hPSC-derived CPCs in vivo and inducing proliferation and cardiovascular differentiation in a drug-regulated manner.
Methods and results
CPCs were injected firstly at a non-cardiac site in Matrigel suspension under the skin of immunocompromised mice to assess their commitment to the cardiovascular lineage and ability to self-renew or differentiate in vivo when instructed by systemically delivered factors including DOX and basic fibroblast growth factor (bFGF). CPCs in Matrigel were then injected intra-myocardially in mice subjected to MI to assess whether expandable CPCs could mediate cardiac repair. Transplanted CPCs expanded robustly both subcutis and in the myocardium using the same DOX/growth factor inducing regime. Upon withdrawal of these cell-renewal factors, CPCs differentiated with high efficiency at both sites into the major cardiac lineages including CMs, endothelial cells, and smooth muscle cells. After MI, engraftment of CPCs in the heart significantly reduced fibrosis in the infarcted area and prevented left ventricular remodelling, although cardiac function determined by magnetic resonance imaging was unaltered.
Conclusion
Replacement of large areas of muscle may be required to regenerate the heart of patients following MI. Our human/mouse model demonstrated that proliferating hPSC-CPCs could reduce infarct size and fibrosis resulting in formation of large grafts. Importantly, the results suggested that expanding transplanted cells in situ at the progenitor stage maybe be an effective alternative causing less tissue damage than injection of very large numbers of CMs.
Keywords: Expandable human cardiovascular progenitors, Myocardial infarction, Mouse model, Transplantation, Human pluripotent stem cells
Graphical Abstract
Graphical Abstract.
1. Introduction
Cardiovascular diseases, including those associated with myocardial infarction (MI), are a major and enduring cause of death worldwide. MI results in the loss of up to 1 billion contractile cardiomyocytes (CMs) and since these divide only slowly in adult heart, their early replacement has been proposed as an approach to prevent later heart failure.1 Human pluripotent stem cells (hPSCs) are already being actively explored as a source of replacement cardiac cells, since they are now widely available and can be derived efficiently in large numbers in bioreactors.2 However, washout from the transplantation site and low survival can limit graft size, whilst inappropriate coupling with native (resident) CMs can cause arrhythmias.3 Cell types with little cardiomyogenic capacity (like bone-marrow stromal cells) have also been examined both in animal models and patients as alternatives and although demonstrated as safe, they are not retained in the heart for more than a few days and meta-analysis of multiple large-scale clinical trials has not indicated clinically significant improvements in cardiac function.4
While the transplantation of hPSC-derived CMs after MI is among the more promising approaches to restoring contractile function of the heart, the issue of graft size and revascularization are two hurdles that may be difficult to overcome with fully differentiated cells. With their higher proliferative potential and multipotent capacity, cardiovascular progenitor cells (CPCs) may be an effective cell source. However, while CPCs can be isolated from differentiating hPSCs, the challenge of controlling their expansion and differentiation remains.5,6
We previously reported the derivation of CPCs from a human embryonic stem cell (hESC) line containing an NKX2.5eGFP/w reporter in which we had inserted a doxycycline (DOX)-inducible MYC (Tet-On-MYC) construct.5 CPCs derived from this Tet-On-MYC NKX2.5eGFP/w hESC line could be expanded in vitro by mitogen stimulation in the presence of DOX. Upon DOX removal, the CPCs responded predictably to developmental signalling pathways and could undergo highly efficient myocardial and vascular differentiation. Here, we report that this ‘switchable’ system can operate similarly in vivo; DOX in the presence of mitogens induced CPC proliferation in mice to produce large grafts in situ, and after DOX removal cardiac cell differentiation was induced in both cardiac and extra-cardiac sites. In the heart, after MI, cell grafts improved left ventricular thickness and decreased fibrosis.
2. Methods
2.1 CPC culture
To generate CPCs, Tet-On-MYC NKX2.5eGFP/w hESCs were differentiated as embryoid bodies (EBs) as previously described.5 At Day 6, EBs were dissociated using TrypLE Select (Invitrogen, Carlsbad, CA, USA) and cryopreserved in BPEL medium containing 1 μg/mL DOX (Sigma-Aldrich, St. Louis, MO, USA), 5 μM SB431542 (Sigma-Aldrich), 100 ng/mL LONG R3 IGF-1 (Sigma-Aldrich), 1 μM SAG (Millipore, Burlington, MS, USA), 1 ng/mL bFGF (Miltenyi Biotech, Bergisch Gladbach, Germany), 20 ng/mL BMP4 (R&D, Minneapolis, MN, USA) (hereafter called CPC medium) plus 10% DMSO, and 1 µM fasudil. Thawed CPCs were expanded in CPC medium on CorningTM MatrigelTM GFR (growth factor reduced) MembraneMatrix (354 277, BD Bioscience) for 5 days before transplantation. GFP expression was verified by FACS (Supplementary material online, Figure S1).
2.2 In vivo expansion and differentiation of CPCs in immuno-deficient mice
Eight to nine-week-old male NSG mice (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ, Charles River, MA, USA) were used for subcutaneous and intra-myocardial CPC injections. Animal experiments were approved by the Leiden University Medical Center (LUMC) Animal Ethics Committee (protocol n. 13162). The LUMC is an institutional license holder according to Dutch Law on animal experimentation and to the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes. See Supplementary material online, Methods for dosage and regimen of DOX and growth factors.
2.3 Subcutaneous injection of CPCs in NSG mice
Approximately 1000 spheres (500 000 CPCs) in CPC medium (150 µL) were mixed with 10% Corning™ Matrigel™ GFR (growth factor reduced) Membrane Matrix (354 277, BD Bioscience) (150 µL), and subcutaneously injected into the right and left flank area of each mouse.
2.4 Induction of MI in NSG mice and intra-myocardial injection of CPCs
MI was performed as previously reported7 (see Supplementary material online, Methods). Approximately 1000 cardiospheres (500 000 CPCs) were suspended in a total of 50 µL of CPC medium containing 10% Matrigel and injected directly into three points of the surrounding border zone. An overview of all animals used for the cardiac experiments is given in Supplementary material online, Table S1.
2.5 Cardiac magnetic resonance imaging and left ventricular wall thickness evaluation
Cardiac magnetic resonance imaging (MRI) was performed under general anaesthesia and using the methodology further explained in the Supplementary material online. Left ventricular wall thickness was calculated using Mass4mice.8 Briefly, the right ventricular insertion point was marked in all the image slices, from basal to apex, and the left ventricle (LV) was divided into 12 segments (clockwise) per slice, where segments 1–8 correspond to the lateral wall and 9–12 to the septal wall. Segments containing the graft were identified histologically using a staining for β1-integrin and matched with the segments provided in Mass4mice images along with all the slices. One-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test was applied for differences in means between groups. Statistical significance was defined as P < 0.05. MRI investigators were blinded to the treatment groups, both during image acquisition and analysis.
2.6 Isolation and histology of CPC plugs and hearts
Animals were euthanized by CO2; CPC plugs or removed hearts were isolated and fixed for cryosectioning for 4 h in 4% neutral buffered formaldehyde at room temperature (RT), then 2 h in 15% sucrose-phosphate buffered saline (PBS) (RT) followed by overnight 30% sucrose-PBS solution (4°C) before embedding in Tissue-Tek® OCT compound (Sakura® Finetek) at −80°C. Eight-micrometre serial sections (1:10) were prepared for all the samples.
2.7 IF and Sirius Red staining
See Supplementary material online, Methods for information on staining methods. See Supplementary material online, Table S2 for information regarding antibodies used.
2.8 Imaging
Brightfield and fluorescent slides were scanned on a ‘Panoramic’ MIDI digital scanner (3DHISTECH, Budapest, Hungary) using the provided software ‘Panoramic Viewer’ (3DHISTECH). Additional imaging was performed on a Leica TCS SP8 upright confocal microscope (Leica Microsystems, Wetzlar, Germany).
2.9 Quantification of graft size, fibrosis, cardiac troponin T, smooth muscle actin, and CD31 vessel area
The total graft volume, the proportion of cardiac and smooth muscle areas, as well as the vessel area per cardiac graft were determined as described in Supplementary material online, Methods.
2.10 Statistical analysis
Statistical significance (P < 0.05) was detected by one-way ANOVA or Mann–Whitney test. Statistical analysis was performed using Graph Pad Prism 7.0 software. Data are presented as means ± standard error of the mean. For functional analysis (MRI), one-way ANOVA with Tukey’s multiple comparisons test was applied for differences in means between groups. Statistical significance was defined as P < 0.05.
3. Results
3.1 Human stem cell-derived cardiovascular progenitors expand in vivo after subcutaneous injection
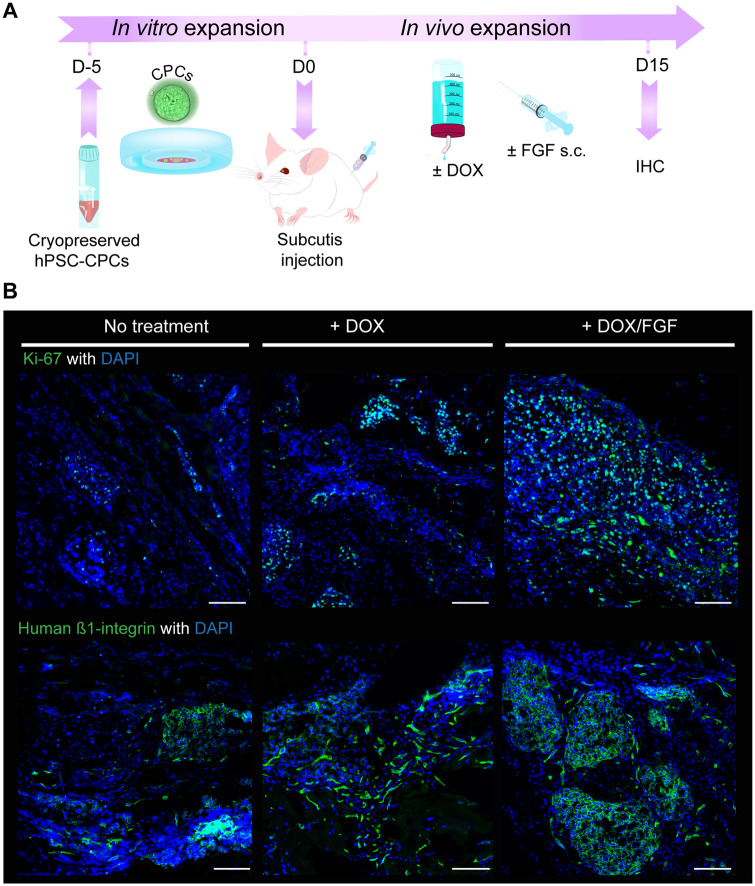
Since DOX-inducible (Tet-On MYC) NKX2.5eGFP+ CPCs can robustly expand in vitro,5 we investigated whether this can also occur in vivo. We first injected in vitro-expanded CPCs under the mouse skin (subcutis), an accessible transplantation site in which the graft size could be monitored over time through palpation without sacrificing the animal (Figure 1A). When assessed by Ki-67 labelling 15 days post injection, transplanted CPCs showed little proliferation in the absence of DOX. However, provision of DOX in the drinking water clearly increased CPC proliferation and this was further enhanced when combined with subcutaneous injection of bFGF (Figure 1B). The human origin of the cells was confirmed by staining for human β1-integrin (Figure 1B). Together these data indicated that transplanted CPCs respond similarly to external cues in vivo as they do in vitro.
Figure 1.
CPCs expand after subcutis injection in vivo. (A) Schematic of the experimental workflow for CPC expansion in vitro and then after subcutaneous injection in mouse. (B) Representative Ki-67 and human β1-integrin immunostaining in plugs from mouse subcutis showing proliferating CPCs; the mice were treated as indicated for 15 days prior to plug removal. Scale bar = 100 µm.
3.2 CPCs differentiate in vivo on DOX withdrawal
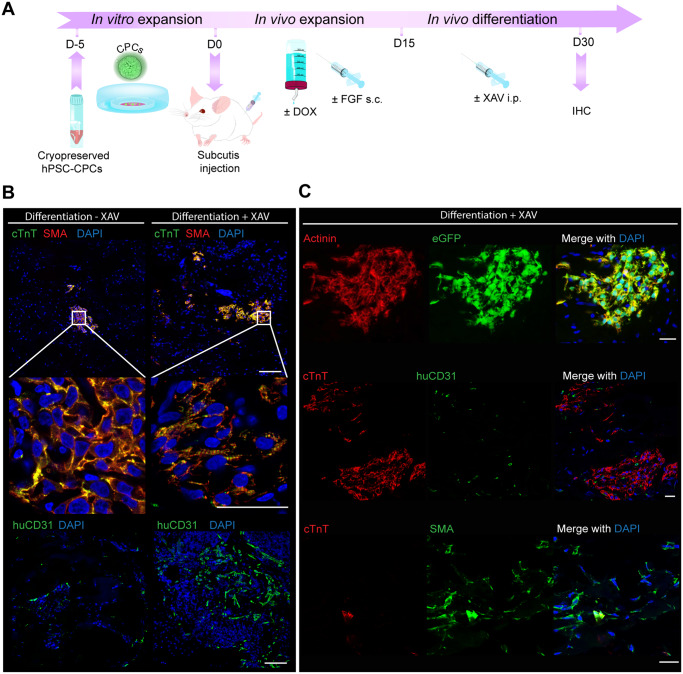
When cultured in vitro, Tet-On MYC NKX2.5eGFP+ CPCs efficiently differentiate to myocardial and vascular cells after withdrawal of DOX.5 Using our subcutaneous in vivo expansion model, a protocol was developed to subsequently induce differentiation, whereby DOX was removed and the mitogenic stimulation by bFGF was decreased by reducing concentration and then stopping delivery (Figure 2A). Injection of the WNT pathway inhibitor XAV939 promoted full differentiation of CPCs to CMs indicated by the formation of a striated cardiac troponin T (cTnT) staining pattern and had a positive effect on PECAM (CD31) endothelial cell (EC) numbers, consistent with a previous report9 (Figure 2B). Following this protocol, tri-lineage cardiac differentiation was thus evident: CMs were detected by endogenous GFP from the NKX2.5eGFP reporter, cTnT and α-actinin; ECs by CD31; and smooth muscle cells (SMC; or activated fibroblasts) by smooth muscle actin (SMA), while being negative for cTnT (Figure 2C). In addition, in a standard teratoma assay, no engraftment of the cells was found in other organs of the mice (Supplementary material online, Figure S2), indicating the presence of MYC had not induced metastatic growth. These results demonstrated that CPCs are capable of safely differentiating towards the main cardiac cell types in vivo even after injection at a non-cardiac site.
Figure 2.
CPC differentiation in vivo promoted by WNT-inhibition after subcutis injection. (A) Schematic of the experimental workflow for CPC expansion after subcutaneous injection in mouse, followed by directed differentiation by intraperitoneal (i.p.) injection of XAV939. (B) Human cTnT, SMA and human-specific CD31 (huCD31) immunostaining in plugs from mouse subcutis after the CPC expansion and differentiation protocol with or without i.p. injection of XAV939; scale bar = 100 µm in upper panel and lower panel; scale bar = 25 µm in middle panel. (C) Additional immunostaining with human-specific antibodies after CPC differentiation in the subcutis in the presence of XAV939: alpha-actinin is shown to co-label with eGFP in CMs; cTnT+ areas are shown with interspersed huCD31+ endothelial cells (ECs); and SMA+ cTnT-labelling indicates the presence of SMCs; scale bar = 25 µm.
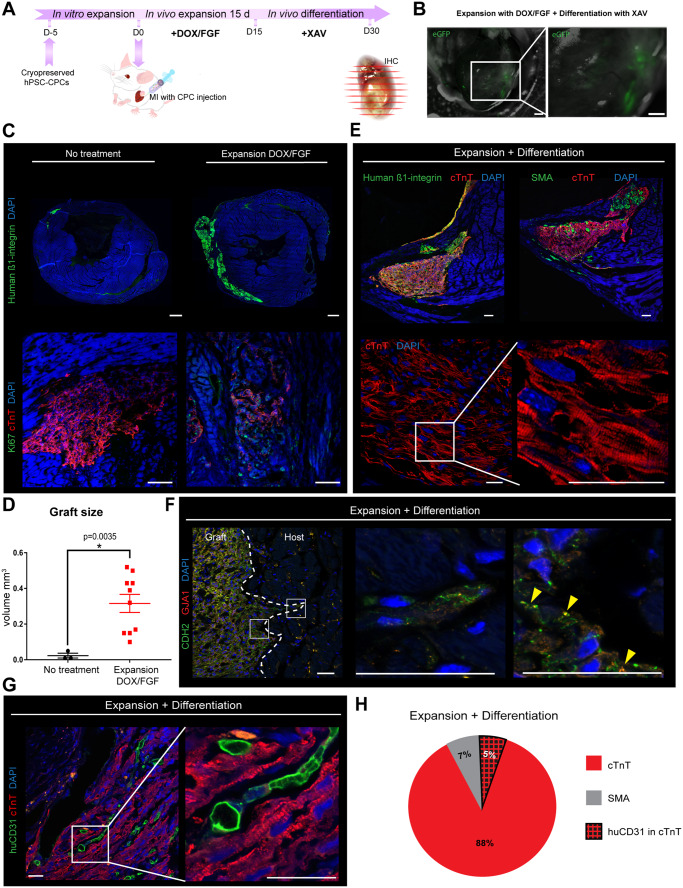
3.3 Heart-injected CPCs expand and form large grafts composed of CMs, ECs, and SMCs after MI in mice
We next investigated whether CPCs could be similarly expanded after transplantation into the heart post-MI induced by permanent ligation of the left ascending coronary artery, and differentiated towards new myocardial and vascular cells. To assess this, CPCs were injected into the border zone of the infarcted myocardium, expanded for 15 days using DOX/bFGF and then differentiated for 15 days in the presence XAV939 as optimized for the subcutis transplantation (Figure 3A) (see Supplementary material online, Table S1 for full experimental conditions). Expanded eGFP+ cells were detectable in hearts by bright field and fluorescent microscopy (Figure 3B). Staining against eGFP confirmed that all GFP+ cells were β1-integrin+ (Supplementary material online, Figure S3). Graft volume, assessed by human β1-integrin labelling, increased from 0.02 ± 0.01 mm3 without treatment to 0.32 ± 0.05 mm3 with DOX/bFGF stimulation (P < 0.05) (Figure 3C and D). Moreover, DOX/bFGF treatment restrained differentiation to cTNT+ cells and maintained CPC proliferation, as observed by Ki-67 expression. Extending DOX/bFGF treatment to 30 days unexpectedly resulted in no further increase in graft size even though there was no apparent increase in apoptosis (Supplementary material online, Figures S4C and S5B). After DOX removal, differentiation of expanded CPCs to CMs was clearly evident, with >80% of the cells in the β1-integrin+-grafts being cTnT+ (Figure 3E). The cTnT-negative graft area, representing 7 ± 6% of the graft area, stained positively for SMA, suggesting that these cells are SMCs or activated myofibroblasts (Figure 3E). Despite this, by examining gap junctions we found no evidence of coupling between the host tissue and the graft (Figure 3F). We confirmed that after DOX removal the MYC transgene was switched off, demonstrating the robustness of the system (Supplementary material online, Figure S5H). Also, as in the subcutaneous injections, staining for human-specific CD31 revealed that CPCs have the potential to differentiate into blood vessel ECs (Figure 3G). Quantification of the CD31+ pixels per graft showed that about 5.4 ± 2% of the cardiac graft was vascularized by human ECs (Figure 3H). Similar EC incidence was observed when differentiation was induced after 30 days of DOX/bFGF exposure (Supplementary material online, Figure S4). Together, CPCs appeared to have efficiently differentiated into cTnT+ CMs, CD31+ ECs, as well as SMA+ SMCs, at frequencies similar to those previously observed in vitro.5
Figure 3.
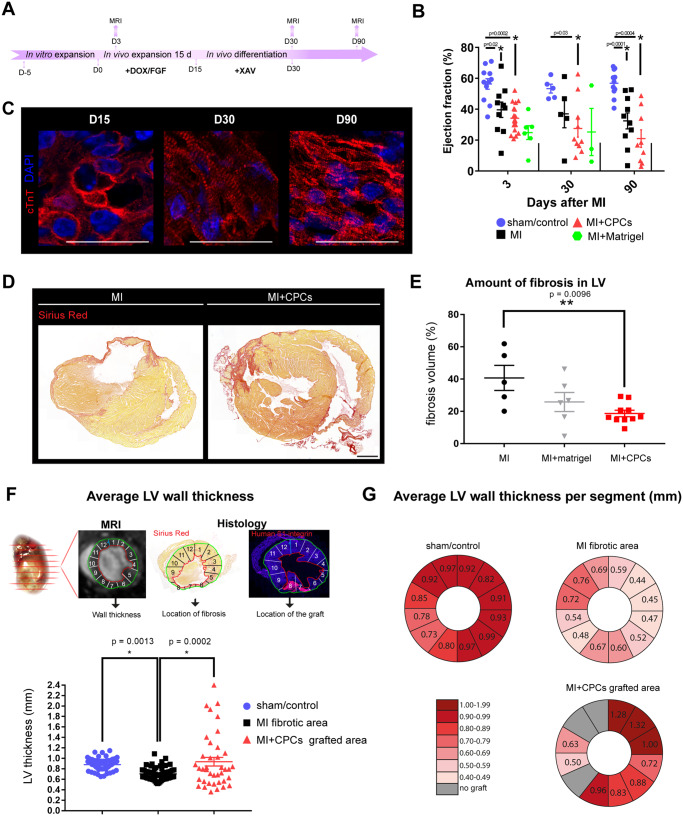
CPCs form large grafts composed of CMs, ECs, and SMCs after intra-myocardial injection post-MI in vivo. (A) Schematic of the experimental workflow for intra-myocardial CPC injection, expansion, and differentiation after MI. (B) Visualization of infarct area by bright field microscopy and intra-myocardial grafts by endogenous GFP (eGFP); infarct area marked in red; scale bar = 500 µm. (C) Representative images of grafts visualized by human β1-integrin staining and DAPI after no treatment and DOX/bFGF treatment, scale bar = 500 µm; Ki-67 expression (green) and cTnT (red) show that DOX/bFGF promotes expansion by proliferation of the CPCs, scale bar = 75 µm. (D) Quantification of graft volume; data are expressed as means ± SEM; one-way ANOVA with Tukey’s multiple comparisons test was applied for differences in means between groups. Statistical significance was defined as P < 0.05; n = 3 for no treatment, n = 10 for DOX/bFGF treatment. (E) Representative confocal immunofluorescent pictures of grafts stained for human β1-integrin (green), cTnT (red), SMA (green), and DAPI (blue); scale bar = 75 µm. High-resolution images of cTnT shows alignment of the sarcomeres in the graft. Scale bar = 25 µm. (F) Representative confocal picture showing the engraftment of the human cells in the mouse heart. Staining for the gap junction proteins GJA1 (connexin 43, red) and N-cadherin (CDH2, green); scale bar = 25 µm. (G) Representative confocal pictures of hearts stained for the human-specific endothelial cell marker CD31 (huCD31, green), cTnT (red), and DAPI (blue); scale bar = 25 µm. (H) Pie chart to illustrate the average cell composition of the grafts after differentiation.
3.4 CPC grafts did not improve cardiac function but diminished fibrosis and left ventricular remodelling after MI
However, an important question was whether CPC expansion and differentiation in vivo affected cardiac function, fibrosis, and left ventricular remodelling (Figure 4A). As early as 3 days following MI in mice, a significant reduction in ejection fraction was detected compared to sham-treated mice; however, there was no significant difference between mice with MI with or without injected CPCs or Matrigel-only, suggesting that the injection of CPCs did not improve cardiac function following acute MI despite the presence of large grafts (Figure 4B and Supplementary material online, Figure S6A). Similarly, we observed no improvement of cardiac function at the endpoint of the experiment after 90 days despite evident structural CM maturation over these 90 days in vivo (Figure 4C). MI caused the formation of large fibrotic scars detectable by Sirius Red staining which encompassed 41 ± 13% of the left ventricular volume. The injection of CPCs significantly diminished the extent of fibrosis by more than half to 20 ± 6% (P < 0.05) (Figure 4D and E and Supplementary material online, Figure S6C). To evaluate the impact on left ventricular remodelling, we assessed left ventricular wall thickness by cardiac MRI in a 12-segment model of the LV (Figure 4E). Average wall thickness was reduced from 0.88 ± 0.11 mm in sham mice to 0.70 ± 0.1 mm in mice with acute MI (P < 0.05) and was restored to 0.94 ± 0.5 mm mice with MI that had received CPC transplants in segments of the grafted area (Figure 4F). Similarly, average wall thickness per segment was preserved as a result of the transplantation (Figure 4G).
Figure 4.
Injection of CPCs did not improve cardiac function, but significantly reduced fibrosis and left ventricular remodelling after MI. (A) Schematic of the experimental workflow highlighting the time points of MRI and histological evaluation. (B) Quantification of cardiac function by ejection fraction; data are expressed as means ± SEM and individual data points of each animal; one-way ANOVA with Tukey’s multiple comparisons test was applied for differences in means between groups. Statistical significance was defined as P < 0.05. Animal groups are described in Supplementary material online, Table S1. (C) High-magnification confocal pictures representative of human cardiomyocytes at different time points during in vivo differentiation in the mouse heart; scale bar = 25 µm. (D) Representative scans of grafts stained for Sirius Red to visualize cardiac fibrosis in mice with MI only (MI) or MI with injected CPCs (MI + CPCs); scale bar = 1000 µm. (E) Quantification of fibrosis volume in percent of left ventricular volume after MI (n = 5) or MI plus injection of CPCs (MI + CPCs) (n = 10). (F) Schematic of the evaluation of ventricular wall thickness by cardiac MRI in a 12-segment model of the left ventricle. (G) Average wall thickness in the fibrotic area in mice with acute MI (n = 6 mice for a total of N = 81 evaluated segments) and mice with MI plus injection of CPCs (n = 4, N = 40 segments) compared to sham (n = 10, N = 120 segments) in segments of the grafted area. Data points represent all segments per animal. (H) Average wall thickness in each segment of the left ventricle. Data are expressed as mean ± SEM; one-way ANOVA with Tukey’s multiple comparisons test was applied for differences in means between groups. Statistical significance was defined as P < 0.05.
4. Discussion
In this study, we investigated whether hPSC-derived CPCs expressing a DOX-inducible MYC construct could expand and differentiate to cardiovascular cells in vivo in mice with view to investigating whether increased graft size improves cardiac function or remodelling after MI but circumventing challenges like tissue damage and cell effluence that arise from transplanting large cell numbers to the heart. We found that: (i) CPCs expanded in response to DOX-induced MYC together with bFGF signalling, (ii) CPCs differentiated to cardiovascular cells in vivo outside the cardiac microenvironment after systemic administration of WNT-inhibitor XAV939, (iii) large grafts could form in vivo in the heart after transplanting relatively few CPCs which was the result of CPC proliferation in situ, (iv) CPCs could undergo trilineage differentiation in both extra- and intra-myocardial grafts, (v) ultimately graft size may have been limited by available vasculature, and (vi) whilst cardiac function did not improve, there was significant decrease in fibrosis and significant increase of left ventricular thickness as a result of the transplantation.
Unlike hPSC-derived neural progenitors, hPSC-derived CPCs have been difficult to maintain in an undifferentiated state for unknown reasons, and this is a roadblock for achieving meaningful expansion and cell replacement in the heart. Highlighting this problem, Fernandes et al.10 reported smaller grafts from CPCs than from hPSC-CMs and almost no differentiation to ECs. Controlling the phases of CPC self-renewal and differentiation after cell transplantation could be a powerful therapeutic approach. Although not a regulatable system, recently, Zhu et al.11 reported the use of a constitutive CCDN2 transgene, which promoted the expansion of transplanted hPSC-CMs. Here, we used MYC, due to the known role of this family of transcription factors in CPC self-renewal and heart development12 and its power to drive phenotypically silent CMs replacement in mosaic mice.13 Additionally, we controlled its expression and thus the expansion of the cells conditionally using a DOX-inducible system, which proved highly effective. The discovery and use of more specific downstream genes may pave the way towards clinical relevance. Moreover, the delivery of external growth factors (bFGF) and small molecules (XAV939) led to predictable results based on the action of these factors in vitro, proving the value of further defining the signalling requirements of these cells first in culture. A surprising result was that cell expansion was not increased in the heart beyond 15 days of DOX exposure, which suggests a limit may be reached in terms of available vasculature or the stimulus for differentiation may be ultimately too strong. Understanding this will be an important future goal. Our finding that adverse cardiac remodelling and fibrosis were largely prevented in mice with large CPC grafts whereas ejection fraction was not improved, may be explained by the physiological difference between mouse and human CMs, which may act as a hindrance to cell coupling. Previous reports using murine iPSC-derived CPCs have shown functional benefit in a similar MI model (Mauritz et al. 2011).14 In this case, though, Matrigel was not used as a delivery vehicle. In addition, species differences in heart rate, ion channels, and contractile proteins are substantial and our results suggest the evolutionary distance between mice and men may be too great for functional insight; the use of larger animal models may be required. In summary, our model has demonstrated the potential of proliferating hPSC-derived CPCs to repair the heart following MI and creates an exciting opportunity to maximize the therapeutic potential of cardiac cell transplantation by expanding cardiomycytes in situ rather than injecting large cell numbers (in the order of billions) which in themselves can cause significant additional damage. Our use of Matrigel to stabilize vasculature precluded functional improvements but illustrated that connection with host tissue is not essential for reducing fibrosis and increasing wall thickness resulting from the presence of CMs. Advancing our understanding of the cardiac developmental biology will undoubtedly help towards overcoming the use of the MYC transgene and hence achieving clinical success.
Supplementary Material
Acknowledgements
We would particularly like to thank Jantine J. Monshouwer-Kloots for assistance with animal experiments and histology, Ernst Suidegeest for technical help with the MRI, and Christian B. Schwach for illustrations.
Conflict of interest: none declared.
Funding
This work was supported by the Netherlands Heart Foundation [CVON-HUSTCARE 2012-18].
Time for primary review: 29 days
Translational perspective
A billion cardiomyocytes are lost after a typical myocardial infarction. A promising approach to regenerate the damaged heart with new muscle and vasculature is by stem cell therapy. Being multipotent, cardiovascular progenitor cells (CPCs) from human pluripotent stem cells meet the requirements for donor cells. But the mechanisms governing their proliferation are still being investigated and CPC expansion remains challenging. Using a drug-regulated genetic model, this work shows that when transplanted CPCs are programmed to proliferate, they can form larger grafts of new myocardium and vascular cells. Achieving this through physiological means could help deliver effective heart regeneration in patients.
References
- 1. Laflamme MA, Murry CE.. Regenerating the heart. Nat Biotechnol 2005;23:845.. [DOI] [PubMed] [Google Scholar]
- 2. Kempf H, Andree B, Zweigerdt R.. Large-scale production of human pluripotent stem cell derived cardiomyocytes. Adv Drug Deliv Rev 2016;96:18–30. [DOI] [PubMed] [Google Scholar]
- 3. Chong JJH, Yang X, Don CW, Minami E, Liu Y-W, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem H-P, Laflamme MA, Murry CE.. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014;510:273.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chong JJH, Murry CE.. Cardiac regeneration using pluripotent stem cells—progression to large animal models. Stem Cell Res 2014;13:654–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Birket MJ, Ribeiro MC, Verkerk AO, Ward D, Leitoguinho AR, den Hartogh SC, Orlova VV, Devalla HD, Schwach V, Bellin M, Passier R, Mummery CL.. Expansion and patterning of cardiovascular progenitors derived from human pluripotent stem cells. Nat Biotechnol 2015;33:970.. [DOI] [PubMed] [Google Scholar]
- 6. Birket MJ, Mummery CL.. Pluripotent stem cell derived cardiovascular progenitors—a developmental perspective. Dev Biol 2015;400:169–179. [DOI] [PubMed] [Google Scholar]
- 7. Winter EM, Grauss RW, Hogers B, van Tuyn J, van der Geest R, Lie-Venema H, Steijn RV, Maas S, deRuiter MC, deVries AAF, Steendijk P, Doevendans PA, van der Laarse A, Poelmann RE, Schalij MJ, Atsma DE, Gittenberger-de Groot AC.. Preservation of left ventricular function and attenuation of remodeling after transplantation of human epicardium-dervied cells into the infarcted mouse heart. Circulation 2007;116:917–927. [DOI] [PubMed] [Google Scholar]
- 8. van der Geest RJ, Reiber J.. Quantification in cardiac MRI. J Magn Reson Imaging 1999;10:602–608. [DOI] [PubMed] [Google Scholar]
- 9. Reichman DE, Park L, Man L, Redmond D, Chao K, Harvey RP, Taketo MM, Rosenwaks Z, James D.. Wnt inhibition promotes vascular specification of embryonic cardiac progenitors. Development 2018;145:dev159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fernandes S, Chong JJH, Paige SL, Iwata M, Torok-Storb B, Keller G, Reinecke H, Murry CE.. Comparison of human embryonic stem cell-derived cardiomyocytes, cardiovascular progenitors, and bone marrow mononuclear cells for cardiac repair. Stem Cell Reports 2015;5:753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhu W, Zhao M, Mattapally S, Chen S, Zhang J.. CCND2 overexpression enhances the regenerative potency of human induced pluripotent stem cell-derived cardiomyocytes. Circ Res 2018;122:88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moens CB, Stanton BR, Parada LF, Rossant J.. Defects in heart and lung development in compound heterozygotes for two different targeted mutations at the N-myc locus. Development 1993;119:485–499. [DOI] [PubMed] [Google Scholar]
- 13. Villa del Campo C, Clavería C, Sierra R, Torres M.. Cell competition promotes phenotypically silent cardiomyocyte replacement in the mammalian heart. Cell Rep 2014;8:1741–1751. [DOI] [PubMed] [Google Scholar]
- 14. Mauritz C, Martens A, Rojas SV, Schnick T, Rathert C, Schecker N, Menke S, Glage S, Zweigerdt R, Haverich A, Martin U, Kutschka I.. Induced pluripotent stem cell (iPSC)-derived Flk-1 progenitor cells engraft, differentiate, and improve heart function in a mouse model of acute myocardial infarction. Eur Heart J 2011;32:2634–2641. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.