Abstract
A striking feature of the liverwort Sphaerocarpos is that pairs of male and female spores remain united in permanent tetrads. To identify the nature of this phenomenon and to test the hypothesis that callose is involved, we examined spore wall development in Sphaerocarpos michellii, with emphasis on the appearance, location and fate of callose vis-à-vis construction of the sculptoderm. All stages of sporogenesis were examined using differential interference contrast optics, and aniline blue fluorescence to locate callose. For precise localization, specimens were immunogold labeled with anti-callose antibody and observed in the transmission electron microscope. Callose plays a role in Sphaerocarpos spore wall development not described in any other plant, including other liverworts. A massive callose matrix forms outside of the sculptured sporocyte plasmalemma that predicts spore wall ornamentation. Consequently, layers of exine form across adjacent spores uniting them. Spore wall development occurs entirely within the callose and involves the production of six layers of prolamellae that give rise to single or stacked tripartite lamellae (TPL). Between spores, an anastomosing network of exine layers forms in lieu of intersporal septum development. As sporopollenin assembles on TPL, callose progressively disappears from the inside outward leaving layers of sporopollenin impregnated exine, the sculptoderm, overlying a thick fibrillar intine. This developmental mechanism provides a direct pathway from callose deposition to sculptured exine that does not involve the intermediary primexine found in pollen wall development. The resulting tetrad, encased in a single wall, provides a simple model for development of permanent dyads and tetrads in the earliest fossil plants.
Keywords: (1,3)-β-glucan; Cryptospore; Exine; Paleozoic; Permanent tetrad; Sculptoderm; Spores; Tripartite lamellae; Ultrastructure
Introduction
Nearly one hundred years ago, Allen (1917, 1919) made a historical discovery of morphologically distinct X- and Y-chromosomes in the tiny ephemeral liverwort Sphaerocarpos. This first account of sex chromosomes in a plant paved the way to a new understanding of the genetic control of sex inheritance in a vast array of dioecious plants. An intriguing aspect of the life history of Sphaerocarpos and one that has facilitated continued genetic, physiological and population studies is the unique adherence of two male and two female spores in a permanent tetrad and the consequent production of dimorphic male and female gametophytes from each (McLetchie 1992; McLetchie and Collins 2001). This condition captured the interest of neobotanists and paleobotanists alike and led early workers to refer to tetrads of Sphaerocarpos as “tetraspores” (Petounnikow 1867). Permanent tetrads are relatively infrequent in bryophytes; only the liverworts Sphaerocapos, Cryptothallus and species in Riccia subg. Thallocarpus produce spores that remain united, disperse and even germinate in tetrads. Permanent tetrads and dyads are widespread in ancient terrestrial cryptophytes of the Ordovician and Silurian that produced envelope-enclosed and naked united tetrads and dyads (Edwards et al. 2014; Wellman et al. 2003; Wellman 2004; Taylor 1995a, b; Gray 1985). Cryptospores predated plant macrofossils by some 35 million years; thus, the study of permanent tetrads in liverworts, the first diverging extant land plant group, provides a unique window in which to evaluate spore production during the early colonization of land.
A notable condition of Sphaerocarpos tetrads is that the spore wall ornamentation is continuous across spore boundaries, suggesting sporocytic control of wall development. In a select group of rather disparate liverworts, sculptoderm patterning has been shown to be under the control of the diploid nucleus (Brown et al. 1986). Best known from the simple thalloid liverwort Pallavicinia and the unique leafy liverwort Haplomitrium, this process of spore wall sculpturing involves the appearance of a callosic template in the spore mother cell that predicts and guides spore wall deposition following meiosis. Callose is known to play significant roles in both spore and pollen wall patterning across embryophytes, serving as a mold for ornamentation that appears in the tetrad of many tracheophytes. Equally significant is the occurrence of callose around meiospores of Coleochaete and zygotes of Closterium, indicating that an association of callose with meiosis predates the split of land plants from charophycean green algae (Dubois-Tylski 1981).
Because callose is produced by the spore mother cell and involved in spore wall patterning in some liverworts, we tested the hypothesis that callose plays an important role in the development of the spore-uniting tetrad wall in Sphaerocarpos, as suggested by Doyle (1975) but never verified. We examined the appearance and location of callose during sporogenesis of Sphaerocarpos michellii using correlated light, DIC and aniline blue fluorescence microscopy followed by immunogold labeling in the transmission electron microscope. We found that callose plays a crucial and never before observed role in spore wall deposition. We postulate that based on its occurrence in extant algae, callose involvement in sporogenesis was preadapted in early land plants. Because of the simplicity of the developmental pathway leading to permanent tetrads in Sphaerocarpos it is reasonable to speculate that a similar mechanism may have produced permanent tetrads and dyads in at least some of the earliest cryptophytes (Edwards et al. 2014).
Materials and methods
Plant materials
Sphaerocarpos capsules were collected on the university farms of Southern Illinois University Carbondale, IL, as previously reported by Schuette and Johnson (2010). Vouchers were deposited in personal herbaria of Schuette and Renzaglia (#4500).
Aniline blue staining
Callose was localized in freshly collected sporophytes using fluorescence microscopy with aniline blue. Whole capsules of variable sizes and stages of development were incubated for 1 to 24 h in 1% aniline blue in 0.067 M Na2HPO4 (pH 8.5) buffer in the dark followed by three rinses in the same buffer. A cover slip was applied to the stained tissue and light pressure applied to release content and slides were immediately viewed. Controls were made using buffer without aniline blue.
Electron microscopy
Capsules were excised from gametophytic tissue and fixed in 2% (v/v) glutaraldehyde in 0.05 M phosphate buffer, pH 7.2 for 1 h at room temperature followed by three rinses in buffer. The specimens were post-fixed for 10 min in 1% (w/v) OsO4, rinsed three times in distilled water then serially dehydrated in ethanol at room temperature. For scanning electron microscopy, capsules in 100% ethanol were critical-point dried using CO2 as the transitional fluid, mounted on stubs and sputter-coated for 230 s (76.7 nm) with palladium-gold and viewed using a Hitachi S570 scanning electron microscope.
For transmission electron microscopy, capsules in 100% ethanol were infiltrated slowly with LR White or Spurr's resin (Electron Microscopy Sciences, Hatfield, PA, USA) in 25% increments over the course of 8 d. Specimens were sealed in airtight gelatin capsules (LR White) or Beem capsules (Spurr's) and cured at 60°C for 7 d (LR White) or 3 d (Spurr's). Ultrathin sections (70-100 nm) were cut using a diamond knife on a Leica ultramicrotome (Leica Microsystems, Wetzlar, Germany) and collected on 200-mesh nickel grids and allowed to dry for 2 h at room temperature.
Immunogold labeling
Immunogold labeling for callose was carried out in a humid chamber as follows: sections were blocked overnight in 2% (w/v) bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO, USA) dissolved in 0.05 M phosphate buffered saline (PBS); grids were placed on 10μl drops of monoclonal primary (1,3)-ß-glucan antibody (Biosupplies, Bundoora, VIC, Australia) diluted 1:20 with 2% BSA-PBS and left overnight. Controls were prepared by omitting the primary antibody. Grids were rinsed four times with BSA-PBS, 4 min each. Grids were placed on 10μl drops of gold conjugated (10 nm) goat-anti-mouse secondary antibody (Sigma-Aldrich) for 30 min followed by four washes in PBS (4 min each). Labeled grids were rinsed by dipping them several times into nano-pure water then post-stained in 2% aqueous uranyl acetate for 3 min and in Reynolds's lead citrate for 30 s and air-dried. The sections were examined and digital micrographs captured in a Hitachi 7650 TEM.
Measurements were made on all images in median section (no obliquity) of a structure, and only a single measurement was taken per image. Consequently, sample sizes are low but measurements are reproducible.
Results
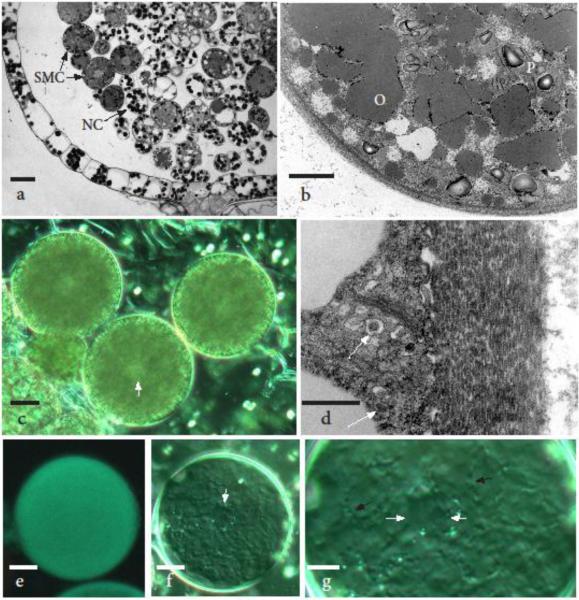
Just prior to the onset of meiosis, sporocytes are round with prominent central nuclei, abundant lipids and dense plastids scattered throughout (Figs. 1a and b). At this stage, sporocytes average 37 μm in diameter (n=22) (Fig. 1c) and do not fluoresce with aniline blue (not shown). Nurse cells are smaller, more vacuolated and contain larger dense starch-filled plastids. The spore mother cell wall averages 0.9 μm thick (n=9) and contains lens-shaped subunits that are deposited by Golgi (Fig. 1d). Aniline blue fluorescence is first visible during meiosis, at which time a callosic wall is deposited between the spore mother cell wall and plasmalemma (Figs. 1e-g). A faint framework of cytoplasmic projections is visible during meiosis around the sporocyte. The pattern of these projections is consistent with the areolate ornamentation that surrounds the tetrad, indicating that wall ornamentation deposited by haploid spores is predicted by the diploid sporocyte.
Fig. 1.
Spore mother cells (SMCs) ranging from 35-55 μm in diameter. a Light micrograph showing the single layered, expanding capsule wall pulled away from the differentiated spore mother cell and nurse cell (NC) mass. The larger spore mother cells have a dense cytoplasm, oil droplets and central nucleus, while nurse cells contain abundant chloroplasts. b Transmission electron micrograph (TEM) of a spore mother cell surrounded by a thin wall and containing fused oil droplets (O), small scattered vacuoles and inconspicuous plastids (P) with starch grains. c Differential interference contrast (DIC) image of three spore mother cells with central nuclei (arrow) that measure less than 40 μm in diameter, are surrounded by a thin wall as in a and b, and do not fluoresce with aniline blue (not shown). d TEM enlargement of spore mother cell wall consisting of lens-shaped units, presumably deposited from Golgi vesicles containing similar electron-dense material (arrows). e First evidence of callose fluorescence is visible in spore mother cells that are approximately 50 μm in diameter. f DIC image of the same SMC as in e demonstrating the presence of a central “nucleus” (arrow) and a faint template made by cytoplasmic projections beneath the developing callose layer. g Enlargement of cell in f showing the cell wall pattern template of cytoplasmic projections (black arrows) and a central meiotic configuration (white arrows) with an outline similar to those in prophase I and metaphase I illustrated in Brown and Lemmon (2012). Scale bars = 35 μm (a), 5.0 μm (b, g), 10 μm (c, e, f ), and 0.5 μm (d)
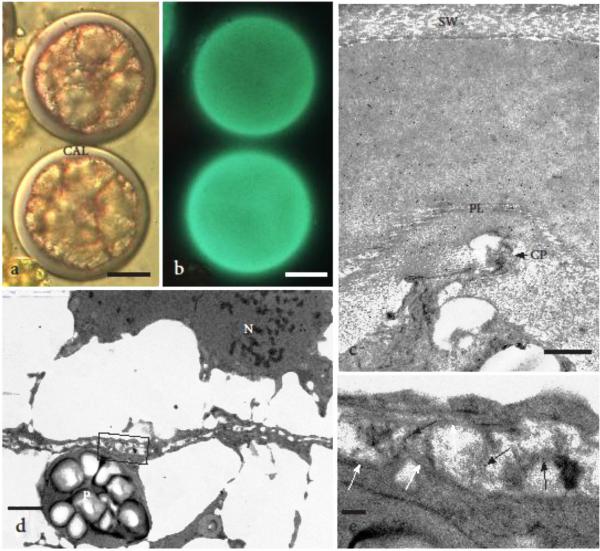
By the onset of cytokinesis, the nascent tetrad averages 65 μm in diameter (n=14) and is surrounded by a thick callosic wall that progressively increases up to 6.2 μm in thickness, fluoresces with aniline blue and labels strongly with the anti-callose antibody (Figs. 2a-c). Prominent cytoplasmic projections are still visible on the distal spore where the ridges (muri) that border areolae in mature spores will form (Fig. 2c). The template for exine deposition is visible around the entire tetrad including over cytoplasmic projections and across spore boundaries. This template consists of first one (Fig. 2c), then two irregular and folded prolamellae embedded in the callosic matrix. The developing intersporal wall forms within a lipid-filled cytoplasm and consists of fused vesicles within which tripartite lamellae (TPL, also known as white-line centered lamellae) differentiate (Fig 2d, e). Compared to distal walls, TPL origin and development in intersporal regions proceed at a faster pace where anti-callose labeling is less dense (Fig. 2e).
Fig. 2.
Newly formed tetrads that measure 60-70 μm in diameter. a DIC image of two young tetrads showing thick callosic sheath (CAL) that surrounds the four spores. b Aniline blue staining of same tetrads as in a showing intense callose fluorescence. c The presence of callose in the distal wall around the tetrad is verified in this TEM by strong gold labeling with anti-callose in the entire special wall regardless of the difference in density. One prolamellar layer (PL) has formed over a prominent cytoplasmic projection (CP) that guides the development of muri in the sporoderm. Original spore mother cell wall (SW) has stretched but still surrounds the round tetrad. d The developing cell plate is indistinct and forms within an oil-laden cytoplasm. Plastid (P) and nucleus (N) are surrounded by oils. Box outlines enlargement in e. e Higher magnification of the portion of the developing proximal wall (cell plate) outlined in d showing beginning of callose (black arrows) and TPL (white arrow) development between spores. Scale bars = 20 μm (a, b), 0.5 μm (c); 1.0 μm (d), and 0.1 μm (e)
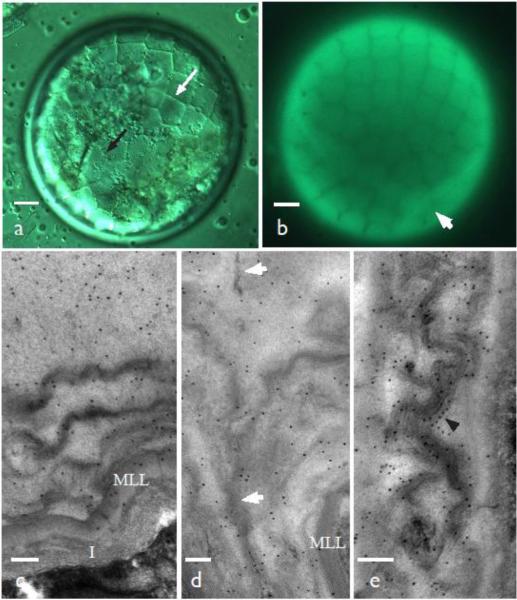
As the tetrad differentiates, it expands in diameter and the pattern of ornamentation is visible under DIC and with aniline blue fluorescence (Figs. 3a, b). Five or six concentric prolamellae within the callose matrix now surround the distal tetrad surface and TPL begin to assemble from each. The outer TPL are solitary while the inner stratum consists of a tightly compacted layer of 6-10 TPL. This multilamellate layer forms the inner boundary of the exine and is subtended by the developing intine that surrounds each spore (Figs. 3c, d). The TPL layers conform to the contours of the mature areolate sculptoderm but at this stage, they are embedded in callose. Anti-callose labeling is abundant throughout the spore wall and is localized on and around developing TPL (Fig. 3c-e). The distal tetrad wall that traverses adjacent spores forms a seam of closely appressed prolamellae/ TPL that are continuous with the intersporal TPL (Fig. 3d). The area between spores, in turn, lacks an intersporal septum and is occupied exclusively by a network of interconnecting stacks of TPL bordered by a multilamellate layer and intine on both spore surfaces (Fig. 3e). Anti-callose labels are particularly uniform along TPL in the intersporal region.
Fig. 3.
Expanding young tetrads 80-90 μm in diameter. a DIC image with areolar exine wall pattern visible within the callosic wall (white arrow) and cytoplasmic projections beneath (black arrow). b Aniline blue fluorescent of same cell showing the exine pattern in the callose. Arrow denotes the junction of three spores. c Strong label with anti-callose antibodies in the distal wall where five or six layers of prolamellae are being replaced by tripartite lamella. A multilamellate layer (MLL) marks the inner limit of the exine, below which is the forming intine (I). d Zone between adjacent spores with similar structure and labelling as in c. Tripartite lamellae are fused along an irregular line that adheres spores (arrows). e The proximal wall of the same stage as c and d has well developed TPL studded with gold labels for callose (arrowhead). This wall forms TPL directly within the callose. Scale bars = 10 μm (a, b), and 100 nm (c-e)
Sporopollenin is deposited on either side of TPL and callose progressively disappears between the TPL/ sporopollenin layers in a centrifugal fashion as the tetrad matures (Fig. 4). Consequently, the sporopollenin is deposited, the sculptoderm takes shape from the inside of the spore outward (Fig. 4b), and callose progressively recedes forming a mold around the exine that is enclosed by the round spore mother cell (Fig. 4b). At this stage, the sculpted callose layer is readily dislodged from the tetrad and can be flattened for viewing (Fig. 4a). Variable levels of aniline blue fluorescence indicate that callose is thickest (brightest fluorescence) and strongest at the junctions between spores or on the distal center of the spore. A continuous seam traverses intersporal regions and is three-parted where three spores meet (Fig. 4b). In the TEM, the seam is irregular and consists of adhering TPL surrounded by sporopollenin (Fig. 4c). The patchy labeling of callose among exine elements illustrates the process of callose dissolution. As the sculptoderm develops, individual spores expand forming a lobed tetrad still surrounded by callose and enclosed in the stretched spore mother cell wall (Fig. 4d).
Fig. 4.
Developing tetrads still within the spore mother cell wall 90-100 μm in diameter begin tetrahedral lobing as callose disappears and spore wall ornamentation develops. a Round tetrads showing light blue fluorescence of the sporopollenin in exine next to the cytoplasm (black arrow), yellow fluorescence of callose layer due to aniline blue (white arrow) and dark outer mother cell wall. b Aniline blue fluorescence of callosic special wall that was pushed out of the spore mother cell wall and flattened making the entire wall visible. Areolate exine pattern is evident in thin areas of callose as is the central papilla in each areole. Weak areas where callosic layer separated are areas where the exine muri are most developed. Callose is thicker (brighter fluorescence) at the juncture of spores and the distal most surface. The trilete line of contact between three spores identified by asterisk (*) and juncture of two spores by arrowheads. c TEM at the distal junction between spores showing strong labeling of callose (inset of upper right corner at 4X higher magnification) outside the exine and isolation of label to patches of electron dense material (arrows) within the layers of TPL. Callose degrades progressively from inside the spores to the outside of the tetrad as sporopollenin assembles on TPL. Arrowheads denote irregular seam in the exine between adjacent spores. d SEM of slightly older tetrads still in the mother cell wall that have assumed tetrahedral lobing due to individual spore enlargement. Arrowhead identifies boundary between two spores. Scale bars = 20 μm (a), 30 μm (b), 0.5μm (c), and 10 μm (d)
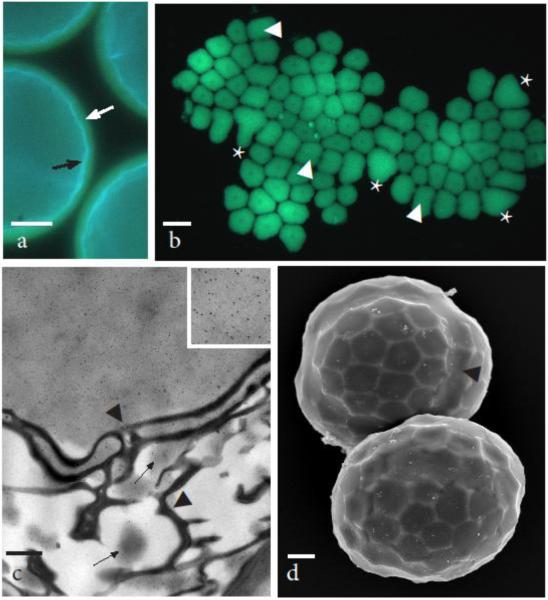
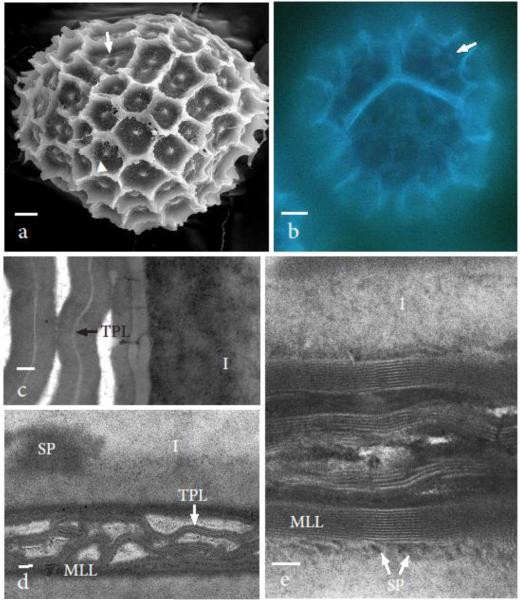
Final stages of tetrad differentiation involve the expansion of the exine, and entire loss of callose and dissolution of the spore mother cell wall (Fig. 5a, b). Most notable in mature tetrads is the continuation of the sculptoderm across spore boundaries in a pattern predetermined during meiosis (Fig. 5a). The tetrad measures 100-120 μm in diameter and contains 6 to 8 aereoles across the distal space of each spore. Areolae measure 7-10 μm in diameter, contain central papillae surrounded by smaller papillae and form spines up to 11 μm high where the areoles intersect. Sporopollenin is deposited from the intine onto the TPL in both distal and proximal surfaces (Fig. 5a-c). The distal exine in mature tetrads does not label with anti-callose antibodies nor with aniline blue (Figs. 5b-d), and contains thick interconnecting layers of sporopollenin within which are TPL. A thick intine that averages 1.0 μm in thickness (n=8) subtends the exine around each spore (Figs. 4c, d) and is overlain by a multilamellate layer up to 11 TPL. Proximally, spores are connected by interconnecting multilamellate layers that eventually become infused with sporopollenin in the mature tetrad (Figs. 5d, e).
Fig. 5.
Nearly mature to mature tetrads have a lobed outer surface range from 100-120 μm in diameter at widest points. a SEM viewed from the distal side of one tetrad. Only remnants of the spore mother cell wall remain (arrowhead). Ornamentation consists of 6-8 areoles 7-10 μm in diameter across the distal surface of each spore. Each areole has a central papilla surrounded by minute papillae. The seam between spores is characterized by continuous half areoles both with central papillae and three fused papillae where the three spores meet (arrow). b Auto fluorescence of tetrad stained with aniline blue shows no callose fluorescence remains and is identical to controls at the same stage (not shown). Autofluorescence of sporopollenin reveals lamellate nature of the proximal and distal walls and central papillae (arrow). c Nearly developed intersporal zone composed of intines on either side of interconnected layers of tripartite lamella (TPL) surrounded by sporopollenin. Intine is two layered with dense areas (SP) bordering inner layer that resemble sporopollenin in density and texture. d Distal wall gold-labeled for callose with no label found. Layers of tripartite lamellae (TPL) surrounded by sporopollenin and an inner multilamellate layer (MLL) comprise the exine. A thick intine (I) surrounds each spore. e Developed intersporal zone composed of interconnected multilamellate layers (MLL) each with 5-11 tripartite lamella surrounded by sporopollenin (SP) that is contributed by the spore cytoplasm through the thick intine (I). Scale bars = 10 μm (a, b), 0.1 μm (c, d), and 50 nm (e)
Discussion
We demonstrate that callose originates early in meiosis and forms a massive matrix (special wall) that encircles the diploid sporocyte of Sphaerocarpos. Spore wall patterning, including the production of a template for exine deposition, appears in the diploid cell within this callosic matrix. The lack of intersporal septa coupled with wall ornamentation that is continuous around the entire tetrad and impregnated with sporopollenin, render the four spores inseparable upon dispersal and germination.
Callose in exine formation in spores and pollen
Callose has been noted during sporogenesis in some bryophytes, however, nothing approaches the prominence of the thick callosic wall that surrounds Sphaerocarpos sporocytes and tetrads. In the liverworts Haplomitrium and Treubia, the two earliest divergent taxa, Pallavicinia, a simple thalloid liverwort, and Nowellia, a leafy liverwort, callose is involved in pre-meiotic determination of spore wall ornamentation, but is restricted to caps or ridges that terminate cytoplasmic projections (Brown et al. 1986; Brown and Lemmon 1987). In these liverworts, slips of TPL and sporopollenin replace callose in the tetrad and the spores separate at maturity (Brown and Lemmon 1990). In Fossombronia wondraczekii, callose was reported to be restricted to the intersporal septum on the proximal surface of young tetrads (Brown and Lemmon 1993). Hornworts and mosses have conflicting reports on the presence of callose during sporogenesis (Brown and Lemmon 1990). Using a range of microscopic approaches, Schuette et al. (2009) provide the only unambiguous report of callose during sporogenesis in a moss, where it is isolated to the expanding aperture region of Physcomitrella patens spores. The callose eventually degrades in mature spores while the aperture collapses. In pteridophytes, callose may or may not be involved in spore development and, as in bryophytes, it is not essential to spore wall development because homosporous lycophytes and eusporangiate ferns lack callose throughout sporogenesis (Lugardon 1990, reviewed in Gabarayeva and Hemsley 2006; Wallace et al. 2011).
Ariizumi and Toriyama (2011) identify three developmental processes that are essential to exine development in pollen; (i) the callose wall forms first around the young tetrad, (ii) primexine is produced, and (iii) sporopollenin is synthesized and transported from the tapetal cells to the primexine, a matrix of glycoproteins and lipopolysaccharides (also called microspore surface coat) (Wallace et al. 2011). In this instance, callose forms a pattern complementary to the mature exine and mediates the development of the primexine in which exine precursors and the sculptoderm develop. In contrast, callose is the material in which TPL and associated sporopollenin assemble and there is no primexine or tapetum in Sphaerocarpos. Based on phylogenomic analyses, Schuette et al. (2009) predicted that a callose synthase homolog PpCalS-5 is responsible for callose production during spore wall development in the moss Physcomitrella. Arabidopsis mutants of CalS-5 produce an aberrant exine, which is attributed to the isolating role of callose and/ or the necessity of this layer for proper primexine development (Dong et al. 2005; Nishikawa et al. 2005; Enns et al. 2005). With the abundance of callose in Sphaerocarpos sporogenesis, it is reasonable to speculate that a CalS-5 gene is present in liverworts even in the absence of primexine.
As in Sphaerocarpos, Physcomitrella does not produce a primexine, yet Schuette (2012) identified a single homolog each of primexine-related DEX (defective in exine formation) and NEX (no exine formation) genes in this moss. Microspores of Arabidopsis that are defective in genes involved in primexine development do not deposit sporopollenin in a normal manner and have an aberrant exine compared with wild type pollen (Paxson-Sowders et al. 1997, 2001; Ariizumi et al. 2004; Guan et al. 2008; Suzuki et al. 2008; Chang et al. 2012). Mutants for primexine production are thought to have limited capacity to bind sporopollenin, thus affecting the outer exine layer (ectexine) that imparts the sculptured pollen surface. Perhaps homologs to genes involved in primexine development in moss play a role in tapetal-derived sporopollenin binding on the spore surface, as has been demonstrated in Arabidopsis primexine mutants. An interesting speculation is that without tapetum and primexine, liverworts may lack some or all of the primexine-related genes, although this awaits publication of a liverwort genome.
Generational control of exine patterning
Similar to what has been described in the liverworts Haplomitrium and Pallavicinia (Brown et al. 1986), a patterned template formed by cytoplasmic wall projections provides visible evidence of spore wall ornamentation in the diploid sporocyte in Sphaerocarpos. The occurrence of these projections brings to mind undulations in the plasma membrane during primexine formation in angiosperms. These undulations are essential to wall sculpturing but unlike Sphaerocarpos, they appear in the tetrad stage of Arabidopsis (Chang et al. 2012). Although no structural evidence for sporophyte control of wall patterning has been found in tracheophytes, diploid inheritance of exine patterning in Arabidopsis is inferred from the exclusive production of microspores with normal primexine from sporophytes that are heterozygous for mutations that disrupt primexine formation (Ariizumi and Toriyama 2011). A logical inference based on this comparison is that evolution of pollen from spores involved a developmental delay in the appearance of wall patterning, still under diploid control, through the interpolation of a primexine in the young microspore.
Relationship between callose deposition and TPL formation
As documented herein, TPL are heavily involved in Sphaerocarpos exine patterning. These structures are present in members of all land plant groups and are generally considered to be the primal form of sporopollenin deposition in spore walls (Wallace et al. 2011). Individual TPL that dictate the sculptoderm of S. michelli spores form within the callose matrix adjacent to the plasmalemma. During this process, anti-callose epitopes are regularly associated with prolamellae and TPL. By the time the intine begins developing, sporopollenin has polymerized on the inner five or six layers of TPL and the callose in this region begins to disappear; thus disappearance of callose occurs from the inside of the developing wall to the outside in a centrifugal manner. Presumably, callase is responsible for callose degradation, as has been shown in seed plants (Gabarayeva and Hemsley 2005). In angiosperms, callase is expressed in tapetal cells and secreted into the locule of the anther, where the enzyme's activity is controlled through regulation of locule pH (Izhar and Frankel 1971; Winiarczyk et al. 2012). The persistence of callose between the outer two TPL during sporopollenin deposition in Sphaerocarpos is consistent with the absence of a tapetum and the contribution of callase from the spore cytoplasm.
Formation of permanent tetrads
A thin callose wall on the proximal surface between adjacent spores forms during cell plate formation in Sphaerocarpos and provides an environment for the prolamellae to form directly and TPL to assemble even before they appear in the distal wall. Callose is known to be involved in cell plate formation from green algae to angiosperms (Scherp et al. 2001) and appears to play a second function in the intersporal region in spore wall deposition. With the molecular machinery for exine production embedded in the callosic wall as it is in the distal callosic wall, the cell plate is equipped to rapidly lay down exine elements. The TPL assemble into multilamellate layers and form an interlocking network connecting adjacent spores and upon which sporopollenin accumulates, visibly contributed by the spore cytoplasm through the intine, while callose progressively disappears. Once formed, such a system precludes separation of individual spores by wall digestion enzymes because there is no intersporal septum to digest, only connected exine elements. In effect, one continuous proximal wall is produced between spores instead of two walls separated by a temporary intersporal septum. In other plants, the intersporal septum is pectinaceous as evidenced by quartet mutants of Arabidopsis in which pectin degradation is blocked during sporogenesis with the result of permanent tetrads (Rhee and Summerville 1998; Rhee et al. 2003; Francis et al. 2006).
It is the combination of the coalesced proximal exine between spores and the development of a continuous wall across spore boundaries that ensure the dispersal and germination of intact spore tetrads. This observation appears at odds with the postulation that the callose layer around microspores in seed plants prevents exine fusion between sister spores (Nishikawa et al. 2005). However, given the position of callose external to primexine in these plants it follows that the absence of callose would juxtapose the primexines of two spores and provide an opportunity for walls to unite, especially during sporopollennin deposition. Because exine elements assemble within callose in Sphaerocarpos, reduction or inhibition of callose synthesis would presumably disrupt all exine production, potentially leading to anomalous thin walls and spore separation.
Permanent tetrads have evolved in many groups of plants, yet the mechanism of spore cohesion is highly variable among these plants. For example, in Selaginella lepidophylla, a thick persistent tetrad envelope surrounds the spores that are detached from each other but fused to the envelope (Morbelli and Lugardon 2012). Compound pollen has evolved several times in flowering plants and is especially prevalent in early-divergent lineages (Taylor et al. 2013). No developmental mechanism that resembles that described here in Sphaerocarpos is seen in any tracheophyte (see review in Taylor et al. 2013), suggesting independent and derived occurrences of permanent tetrads in these plants compared with Sphaerocarpos.
Allen (1923, 1925) identified a mutant strain of S. donnellii in which the spores separated at maturity. Doyle (1975) compared the surface ultrastructures of these spores with that of permanent tetrads to elucidate structural differences between the two. He showed that the exine between adjoining spores is perforated resulting in a weaker adherence whereas exine on the distal surface remains intact. We speculate that the monad strain of Sphaerocarpos may have aberrations in the intersporal callose that is required for normal TPL and sporopollenin deposition along proximal spore walls.
Evolution of permanent dyads and tetrads
The events we document have implications in the role of callose in sporogenesis of the earliest land plants. Because of their close phylogenetic relationship, it is reasonable to speculate that the same mechanism found in Sphaerocarpos occurs in the permanent tetrads of other liverworts such as Riccia and Cryptothallus. If there is a common ontogeny of adherent spores in liverworts, this may well be an ancestral mechanism by which early fossil tetrads were produced. Similarly, if successive not simultaneous cytokinesis occurred, a delay in callose production from early meiosis to the end of the first division would result in two spores encased by a single callosic layer. Given a similar role as callose in wall development in Sphaerocarpos, each pair of spores would remain in permanent, naked (not surrounded by an envelope) dyads, a condition that is widespread in cryptospores (Edwards et al. 2014). Ancient fossil spores were often smooth, lacking elaborate ornamentation, thus callose deposition, followed by TPL and sporopollenin assembly directly at the cell surface from the plasmalemma would be a simple means of ensuring spore adherence. No perine and no deposition of sporopollenin from a tapetum would be required. This process is as rudimentary as any documented in land plant sporogenesis. The occurrence of callose in meiospore walls and zygotes of some charophycean algae (Graham and Taylor 1986; Brown and Lemmon 1990; Graham 1993; Sørensen et al. 2011) supports the existence of callose prior to the divergence of land plants from algae. More importantly, the production of callose in charophytes from diploid and haploid cell participants in meiosis identifies this cell wall polymer as a preadaptation to sporogenesis in land plants and likely involved in the development of the earliest cryptospores. Moreover, the ubiquity of callose during cytokinesis in streptophytes coupled with direct development of exine elements in callose as demonstrated in Sphaerocarpos points to a simple mechanism to connect adjacent spores, one that may help explain the abundance of naked dyads and tetrads in the Paleozoic.
Conclusions
It is becoming apparent that callose is not an impermeable layer preventing interaction from outside to inside the spore, but that it is an active region with unique chemical properties that allow development to occur. In Sphaerocarpos, which has the most extensive callosic wall of all bryophytes, this special polysaccharide wall is a living organelle that is integral to the controlled and patterned development of a complex tetrad wall, one that permanently tethers pairs of male and female spores.
Acknowledgements
We thank Andres Womac, Amelia Merced, and Bryan Piatkowski for technical assistance. This work was supported by NSF grants EF 0531751 and DGE 0638722.
Abbreviations
- MLL
multilamellate layer
- TPL
tripartite lamellae
Footnotes
Authors Contributions
KSR and EEJ conceived, designed and analyzed the data in this study. RAL conducted the immunogold experiments and RAL and EEJ collected TEM micrographs. KSR conducted the aniline blue experiments and collected the DIC and light micrographic data. KSR and EEJ prepared the manuscript. All authors edited and approved the manuscript.
References
- Allen CE. A chromosome difference correlated with sex differences in Sphaerocarpos. Science. 1917;46:466–467. doi: 10.1126/science.46.1193.466. [DOI] [PubMed] [Google Scholar]
- Allen CE. The basis of sex inheritance in Sphærocarpos. P Am Philos Soc. 1919;58:289–316. [Google Scholar]
- Allen CE. Gametophytic inheritance in Sphaerocarpos I. Intraclonal variation, and the inheritance of the tufted character. Genetics. 1924;9:530–587. doi: 10.1093/genetics/9.6.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CE. The inheritance of a pair of sporophytic characters in Sphaerocarpos. Genetics. 1925;10:72–79. doi: 10.1093/genetics/10.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariizumi T, Hatakeyama K, Hinata K, Inatsugi R, Nishida I, Sato S, Kato T, Tabata S, Toriyama K. Disruption of the novel plant protein NEF1 affects lipid accumulation in the plastids of the tapetum and exine formation of pollen, resulting in male sterility in Arabidopsis thaliana. Plant J. 2004;39:170–181. doi: 10.1111/j.1365-313X.2004.02118.x. [DOI] [PubMed] [Google Scholar]
- Ariizumi T, Toriyama K. Genetic regulation of sporopollenin synthesis and pollen exine development. Annu Rev Plant Biol. 2011;62:437–60. doi: 10.1146/annurev-arplant-042809-112312. [DOI] [PubMed] [Google Scholar]
- Brown RC, Lemmon BE, Renzaglia KS. Sporocytic control of spore wall pattern in liverworts. Am J Bot. 1986;73:593–596. [Google Scholar]
- Brown RC, Lemmon BE. Involvement of callose in determination of exine patterning in three hepatics of the sublass Jungermanniidae. Mem New York Botan G. 1987;45:111–121. [Google Scholar]
- Brown RC, Lemmon BE. Sporogenesis in bryophytes. In: Blackmore SB, Knox RB, editors. Microspores: Evolution and ontogeny. Academic Press; London: 1990. pp. 55–94. [Google Scholar]
- Brown RC, Lemmon BE. Spore wall development in the liverwort Fossombronia wondraczekii (Corda) Dum. J Hattori Bot Lab. 1993;74:83–94. [Google Scholar]
- Chang HS, Zhang C, Chang YH, Zhu J, Xu XF, Shi ZH, Zhang XL, Xu L, Huang H, Zhang S, Yang ZN. NO PRIMEXINE AND PLASMA MEMBRANE UNDULATION is essential for primexine deposition and plasma membrane undulation during microsporogenesis in Arabidopsis. Plant Physiol. 2012;158:264–272. doi: 10.1104/pp.111.184853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Hong Z, Sivaramakrishnan M, Mahfouz M, Verma DPS. Callose synthase (CalS5) is required for exine formation during microgametogenesis and for pollen viability in Arabidopsis. Plant J. 2005;42:315–328. doi: 10.1111/j.1365-313X.2005.02379.x. [DOI] [PubMed] [Google Scholar]
- Doyle WT. Spores of Sphaerocarpos donnellii. Bryologist. 1975;78:80–84. [Google Scholar]
- Dubois-Tylski T. Utilisation de fluorochromes pour l'observation des parois cellulaires chez trois especes de Closterium (Desmidiales) au cours de leur reproduction sexuee. Cryptogamie, Algologie. 1981;1:277–287. [Google Scholar]
- Edwards D, Morris JL, Richardson JB, Kenrick P. Cryptospores and cryptophytes reveal hidden diversity in early land floras. New Phytol. 2014;202:50–78. doi: 10.1111/nph.12645. [DOI] [PubMed] [Google Scholar]
- Enns LC, Kanaoka MM, Torii KU, Comai L, Okada K, Cleland RE. Two callose synthases, GSL1 and GSL5, play an essential and redundant role in plant and pollen development and in fertility. Plant Mol Biol. 2005;58:333–349. doi: 10.1007/s11103-005-4526-7. [DOI] [PubMed] [Google Scholar]
- Francis K E, Lam SY, Copenhaver GP. Separation of Arabidopsis pollen tetrads is regulated by QUARTET1, a pectin methylesterase gene. Plant Physiol. 2006;142:1004–1013. doi: 10.1104/pp.106.085274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabarayeva N, Hemsley AR. Merging concepts: the role of self-assembly in the development of pollen wall structure. Rev Palaeobot Palynol. 2006;138:121–139. [Google Scholar]
- Graham LE, Taylor III C. Occurrence and phylogenetic significance of “special walls” at meiosporogenesis in Coleochaete. Am J Bot. 1986;73:597–601. [Google Scholar]
- Graham LE. Origin of land plants. John Wiley & Sons, Inc.; New York: 1993. [Google Scholar]
- Gray J. The microfossil record of early land plants: advances in understanding of early terrestrialization, 1970-1984. Philos T Roy Soc B. 1985;309:167–192. [Google Scholar]
- Guan YF, Huang XY, Zhu J, Gao JF, Zhang HX, Yang ZN. RUPTURED POLLEN GRAIN1, a member of the MtN3/saliva gene family, is crucial for exine pattern formation and cell integrity of microspores in Arabidopsis. Plant Physiol. 2008;147:852–863. doi: 10.1104/pp.108.118026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izhar S, Frankel R. Mechanism of male sterility in Petunia: The relationship between pH, callase activity in the anthers, and the breakdown of the microsporogenesis. Theor Appl Genet. 1971;41:104–108. doi: 10.1007/BF00277751. [DOI] [PubMed] [Google Scholar]
- Lugardon B. Pteridophyte sporogenesis: a survey of spore wall ontogeny and fine structure in a polyphyletic plant group. In: Blackmore S, Knox RB, editors. Microspores: Evolution and ontogeny. Academic Press; London: 1990. pp. 95–120. [Google Scholar]
- McLetchie DN. Sex ratio from germination through maturity and its reproductive consequences in the liverwort Sphaerocarpos texanus. Oecologia. 1992;92:273–278. doi: 10.1007/BF00317375. [DOI] [PubMed] [Google Scholar]
- McLetchie DN, Collins AL. Identification of DNA regions specific to the X and Y chromosomes in Sphaerocarpos texanus. Bryologist. 2001;104:543–547. [Google Scholar]
- Morbelli MA, Lugardon B. Microspore wall organisation and ultrastructure in two species of Selaginella (Lycophyta) producing permanent tetrads. Grana. 2012;51:97–106. [Google Scholar]
- Nishikawa S-I, Zinkl GM, Swanson RJ, Maruyama D, Preuss D. Callose (β-1, 3 glucan) is essential for Arabidopsis pollen wall patterning, but not tube growth. BMC Plant Biol. 2005;5:22. doi: 10.1186/1471-2229-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxson-Sowders DM, Owen HA, Makaroff CA. A comparative ultrastructural analysis of exine pattern development in wild-type Arabidopsis and a mutant defective in pattern formation. Protoplasma. 1997;198:53–65. [Google Scholar]
- Paxson-Sowders DM, Dodrill CH, Owen HA, Makaroff CA. DEX1, a novel plant protein, is required for exine pattern formation during pollen development in Arabidopsis. Plant Physiol. 2001;127:1739–1749. [PMC free article] [PubMed] [Google Scholar]
- Petounnikow A. Sur les organs reproduceors du Sphaerocarpus terrestris. B Soc Bot Fr. 1867;14:137–142. [Google Scholar]
- Renzaglia KS, Schuette S, Duff J, Ligrone R, Shaw AJ, Mishler BD, Duckett JG. Bryophyte phylogeny: advancing the molecular and morphological frontiers. Bryologist. 2007;110:179–213. [Google Scholar]
- Rhee SY, Somerville CR. Tetrad pollen formation in quartet mutants of Arabidopsis thaliana is associated with persistence of pectic polysaccharides of the pollen mother cell wall. Plant J. 1998;15:79–88. doi: 10.1046/j.1365-313x.1998.00183.x. [DOI] [PubMed] [Google Scholar]
- Rhee SY, Osborne E, Poindexter PD, Somerville CR. Microspore separation in the quartet3 mutants of Arabidopsis is impaired by a defect in a developmentally regulated polygalacturonase required for pollen mother cell wall degradation. Plant Physiol. 2003;133:1170–1180. doi: 10.1104/pp.103.028266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherp P, Grotha R, Kutschera U. Occurrence and phylogenetic significance of cytokinesis-related callose in green algae, bryophytes, ferns and seed plants. Plant Cell Rep. 2001;20:143–149. doi: 10.1007/s002990000301. [DOI] [PubMed] [Google Scholar]
- Schuette S. Dissertation. Southern Illinois University; Carbondale: 2012. Ultrastructure, immunocytochemistry, and bioinformatics of spore development in the moss Physcomitrella and the hornwort Dendroceros. [Google Scholar]
- Schuette S, Johnson E. Sphaerocarpos michelii Bellardi (Sphaerocarpaceae) new in Illinois. Evansia. 2010;27:34–35. [Google Scholar]
- Schuette S, Wood AJ, Geisler M, Geisler-Lee J, Ligrone R, Renzaglia KS. Novel localization of callose in the spores of Physcomitrella patens and phylogenomics of the callose synthase gene family. Ann Bot-London. 2009;103:749–756. doi: 10.1093/aob/mcn268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen I, Pettolino FA, Bacic A, Ralph J, Fachuang L, O’Neill MA, Fei Z, Rose JKC, Domozych DS, Willats WGT. The charophycean green algae provide insights into the early origins of plant cell walls. Plant J. 2011;68:201–211. doi: 10.1111/j.1365-313X.2011.04686.x. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Masaoka K, Nishi M, Nakamura K, Ishiguro S. Identification of kaonashi mutants showing abnormal pollen exine structure in Arabidopsis thaliana. Plant Cell Physiol. 2008;49:1465–1477. doi: 10.1093/pcp/pcn131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WA. Ultrastructure of Tetrahedraletes medinensis (Strother and Traverse) Wellman and Richardson, from the upper Ordovician of southern Ohio. Rev Paleobot Palyno. 1995a;85:183–187. [Google Scholar]
- Taylor WA. Spores in earliest land plants. Nature. 1995b;373:391–392. [Google Scholar]
- Taylor ML, Hudson PJ, Rigg JM, Strandquist JN, Green JS, Thiemann TC, Osborn JM. Pollen ontogeny in Victoria (Nymphaeles). J Plant Sci. 2013;174:1259–1276. [Google Scholar]
- Wallace S, Fleming A, Wellman CH, Beerling DJ. Evolutionary development of the plant spore and pollen wall. AoB Plants plr. 2011;027 doi: 10.1093/aobpla/plr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman CH, Osterloff P, Mohluddin U. Fragments of the earliest land plants. Nature. 2003;425:282–283. doi: 10.1038/nature01884. [DOI] [PubMed] [Google Scholar]
- Wellman CH. Origin, function and development of the spore wall in early land plants. In: Hemsley A, Poole I, editors. The evolution of plant physiology. Elsevier; Oxford: 2004. pp. 43–63. [Google Scholar]
- Winiarczyk K, Jaroszuk-Ściseł J, Kupisz K. Characterization of callase (β-1, 3-d-glucanase) activity during microsporogenesis in the sterile anthers of Allium sativum L. and the fertile anthers of A. atropurpureum. Sex Plant Reprod. 2012;25:123–131. doi: 10.1007/s00497-012-0184-5. [DOI] [PMC free article] [PubMed] [Google Scholar]