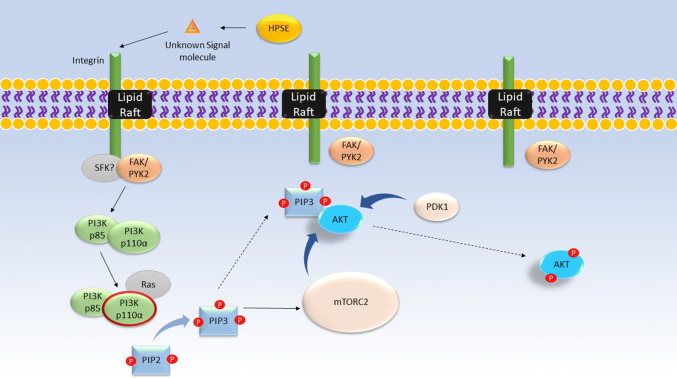
Fig. 1.
Proposed mechanism by which HPSE activates Akt phosphorylation. Proteins shown in gray have roles that are not fully understood. HPSE uses an unknown signaling molecule to bind and activate an integrin bound to a lipid raft. The proteins FAK or PYK2, with a possible role for a Src family kinase, activate the p85 subunit of PI3K. The p85 subunit activates the p110α subunit of PI3K, possibly with the aid of Ras. Activation of PI3K results in the phosphorylation of PIP2 to PIP3. PIP3 plays a dual role in recruiting Akt to the plasma membrane and activating mTORC2. PDK1 and mTORC2 phosphorylate Akt at its Thr-308 and Ser-473 sites, respectively. Phosphorylation of both sites is necessary for maximal activation of Akt