Table 5. Screening of Reaction Conditions for the 1,1-Difluoroolefination of N-Tosylhydrazonea.
| entry | change from standard conditions | yieldb |
|---|---|---|
| 1 | none | 70% |
| 2 | PC (2 mol %) | 77% (73%)c |
| 3 | one-pot, PC (2 mol %) | 75%d |
| 4 | without Cs2CO3 | n.d. |
| 5 | without PC | n.d. |
| 6 | in the dark | n.d. |
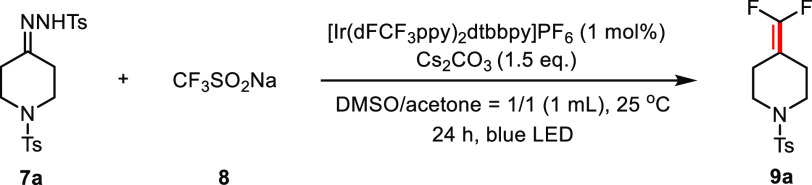
Reaction conditions: compound 7a (0.2 mmol), 8 (0.3 mmol), Cs2CO3 (0.3 mmol), [Ir(dFCF3ppy)2dtbbpy]PF6 (1 mol %) in 1 mL of solvent, irradiation with blue LED (455 nm) at 25 °C for 24 h. n.d. = not detected.
Yields were determined by 19F NMR analysis of the crude reaction mixture using 4,4′-difluorobenzophenone as the internal standard.
Isolated yield.
7a was formed in one pot starting from corresponding ketone and used directly without purification. PC = photocatalyst.