Figure 2.
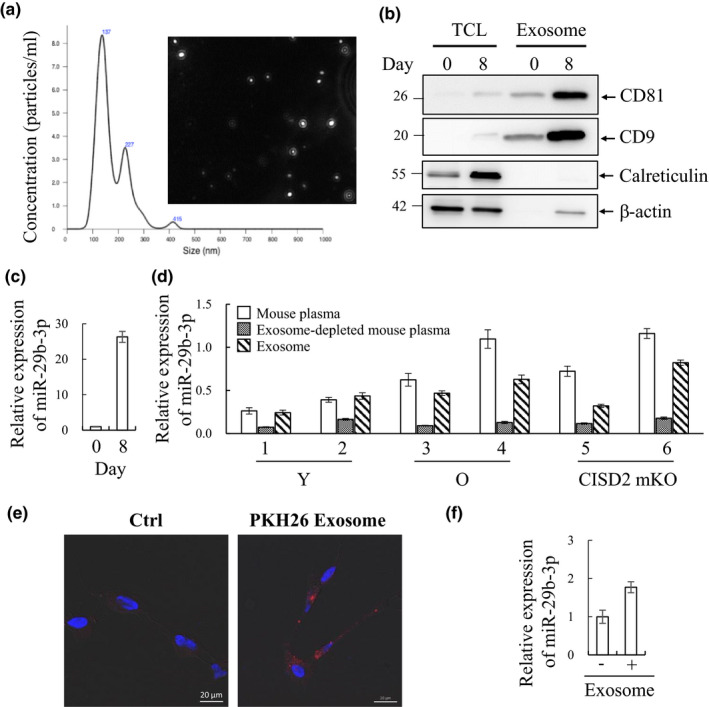
C2C12 myotube‐derived exosomes are taken up by RA‐differentiated SH‐SY5Y cells and results in increased level of miR‐29b‐3p. (a) Exosomes isolated by differential ultracentrifugation were analyzed using nanoparticle tracking analysis (NTA) and represented as size versus. concentration. (b) Immunoblotting assessing the exosome markers, CD81 and CD9, in C2C12 myotube‐derived exosomes enriched from undifferentiated (day 0) or 8 days differentiated cells. The exosome fraction is absent of endoplasmic reticulum (ER) marker calreticulin. β‐Actin was used as loading control. TCL, total cell lysate. (c) Exosomal RNA was purified by TRIzol, and miR‐29b‐3p quantity was determined by RT‐qPCR. (d) RNA from mouse plasma, exosome, and exosome‐depleted plasma was purified by TRIzol, and miR‐29b‐3p quantity was determined by RT‐qPCR. (e) SH‐SY5Y cells were induced for differentiation with 10 μM retinoic acid (RA) for 72 hr. RA‐differentiated SH‐SY5Y cells were maintained in the absence (left) or presence (right) of PKH26‐labeled exosomes (red). 24 hr after co‐incubation, cells were fixed, stained, and visualized by confocal (63x) microscope, demonstrating the presence of exosomes within cells. (f) RNA from RA‐differentiated SH‐SY5Y cells co‐cultured with or without long‐term differentiated C2C12 myotube‐derived exosomes was purified by TRIzol, and miR‐29b‐3p quantity was determined by RT‐qPCR. Error bars show mean ± SD (n = 3)
