Abstract
For cochlear-implant users with near-normal contralateral hearing, a mismatch between the frequency-to-place mapping in the two ears could produce a suboptimal performance. This study assesses tonotopic matches via binaural interactions. Dynamic interaural time-difference sensitivity was measured using bandpass-filtered pulse trains at different rates in the acoustic and implanted ear, creating binaural envelope beats. Sensitivity to beats should peak when the same tonotopic region is stimulated in both ears. All nine participants detected dynamic interaural timing differences and demonstrated some frequency selectivity. This method provides a guide to frequency-to-place mapping without compensation for inherent latency differences between the acoustic and implanted ears.
I. INTRODUCTION
Cochlear implantation has become a viable treatment option for people with unilateral hearing loss, or single-sided deafness (SSD), leading to a population of patients with residual hearing in one ear and a cochlear implant (CI) in the other (SSD+CI). The addition of a CI generally improves localization acuity (Litovsky et al., 2019), although the improvement is based on interaural level differences rather than interaural timing differences (ITDs) in the stimulus envelope or fine structure (Dirks et al., 2019). The addition of the CI can also improve speech perception in spatial listening environments, although the benefits appear to be primarily due to head-shadow or better-ear effects rather than binaural interactions between the acoustic and implanted ears (Williges et al., 2019).
The lack of interactions between the acoustic and implanted ear may reflect fundamental differences in the nature of stimulation or may simply reflect a mismatch between the tonotopic maps of two ears, something commonly observed in this population (Landsberger et al., 2015; Wess et al., 2017). Vocoder simulations of frequency-to-place mismatch in cases of SSD+CI have demonstrated systematic declines in the performance on spatial hearing tasks with increasing interaural frequency mismatch (Wess et al., 2017). One common approach is to combine CT images of electrode arrays in the cochlea with tonotopic maps of spiral ganglion cells (Stakhovskaya et al., 2007). This approach exposes patients to additional, albeit small, doses of radiation and is not sensitive to neural health (i.e., dead regions). Pitch matching has been used to assess tonotopic mismatches between the CI and acoustic ears (Reiss et al., 2015) but the results can be influenced by various non-sensory biases (Carlyon et al., 2010) and may change over time, suggesting perceptual plasticity in pitch judgments (Reiss et al., 2015). Sensitivity to binaural interactions is thought to reflect processes earlier in the auditory pathways, which may be less susceptible to neural plasticity (Hu and Dietz, 2015). Tests of binaural ITD sensitivity in SSD+CI patients have been proposed as a method to determine a tonotopic match (Bernstein et al., 2018; Francart et al., 2018). However, these methods require the determination of a baseline ITD to correct for any frequency-dependent interaural latency differences between the acoustic and implanted ear (Zirn et al., 2015). To overcome this challenge, we propose the use of continuously varying dynamic envelope ITDs, which eliminate the need to account for any baseline latency differences between the ears.
Binaural beats refer to the sensation produced when two low-frequency tones of slightly different frequency are presented to opposite ears. Due to the difference in frequency, the interaural phase relationship in the temporal fine structure between the tones changes over time, cycling through all phase relationships with a repetition rate that corresponds to the difference in frequency between the two tones (Licklider et al., 1950; Perrott and Musicant, 1977). Binaural sensitivity to phase differences between pure tones does not extend beyond 1.5 kHz (Brughera et al., 2013) and CIs remove temporal fine structure during processing. Therefore, to test binaural sensitivity in SSD+CI patients, we used small differences in the rate of acoustic or electric pulses presented to the two ears, thus producing binaural temporal-envelope beats (McFadden and Pasanen, 1975). We first determined whether SSD+CI listeners could detect binaural temporal-envelope beats using broadband acoustic pulse trains. We then bandpass-filtered the acoustic pulse trains to test whether sensitivity varied as a function of the relative place of stimulation between ears. We hypothesized that binaural interactions would be most salient when the place of stimulation in the CI and acoustic ear are best matched.
II. METHODS
A. Participants
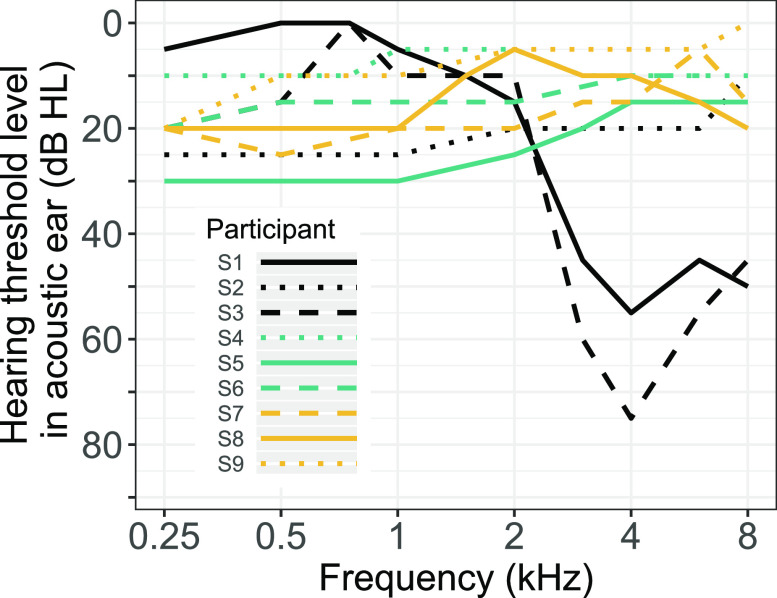
Nine listeners took part in the experiments (5 female, 4 male). They had one ear with adult-onset severe-to-profound sensorineural hearing loss that had been implanted (the CI ear). Most had normal or near-normal hearing across audiometric frequencies in the ear without a CI (acoustic ear), although S1 and S3 had moderate high-frequency hearing loss in the acoustic ear. The listeners' audiograms appear in Fig. 1. Eight of the participants used MED-EL (MED-EL Corp; Durham, SC) devices and one used a Cochlear (Cochlear Corp.; Englewood, CO) device (see Table I for details). All experimental protocols were approved by the Institutional Review Board of the University of Minnesota and all listeners provided informed written consent prior to participation and were paid for their time.
FIG. 1.
(Color online.) Audiograms of the acoustic ear in participants with unilateral hearing loss and a CI in the contralateral ear. Line color and type differentiate individual subjects. In general, listeners were tested at octave frequencies between 250 and 8000 Hz; a subset of listeners were also tested at 750 Hz (S1, S3, and S4) and 1500 Hz (S1, S4, and S8).
TABLE I.
Listener demographics. DoHL represents duration of hearing loss prior to activation. IE represents implanted ear. DoCI represents duration of CI listening experience. The final column reports the three electrodes and corresponding CFs that were tested in each listener.
| Subject | Age | Etiology | DoHL | IE | DoCI | CI | Electrode Number (CF in Hz) |
|---|---|---|---|---|---|---|---|
| S1 | 53 | Unknown | 1 year, 4 months | R | 1 year, 8 months | MED-EL Synchrony, Flex 28, Rondo 1, FS4 | 6 (1182), 8 (2227), 10 (4085) |
| S2 | 37 | Unknown | >10 years | R | 5 years, 5 months | MED-EL Concerto, Flex 24, Rondo 1, FSP | 5 (879), 7 (1746), 9 (3339) |
| S3 | 68 | Otosclerosis | 7 months | R | 2 years, 5 months | MED-EL Synchrony, Flex 28, Rondo 1, FS4-p | 5 (851), 7 (1632), 9 (3064) |
| S4 | 65 | Unknown | 2 years | R | 3 years, 4 months | MED-EL Synchrony, Flex 28, Rondo 1, FS4-p | 4 (653), 7 (1977), 9 (3858) |
| S5 | 44 | Unknown | 10 months | R | 4 years, 9 months | MED-EL Concerto, Flex 28, Rondo 1, FS4 | 5 (1182), 7 (2227), 9 (4085) |
| S6 | 43 | Unknown | 1 years, 4 months | L | 4 yrs, 6 months | MED-EL Concerto, Flex 24, Rondo 1, FS4-p | 5 (971), 7 (1977), 9 (3858) |
| S7 | 51 | Acoustic neuroma | 5 years 10 months | R | 3 yrs, 10 months | MED-EL Concerto, Flex 28, Sonnet 1, FS4 | 5 (971), 7 (1977), 9 (3858) |
| S8 | 39 | Meningitis | 6 months | L | 4 yrs, 3 months | Cochlear Nucleus CI422, CP910, ACE | 13 (930), 9 (1992), 5 (3863) |
| S9 | 35 | Acoustic neuroma | 4 months | L | 2 years | MED-EL Synchrony, Flex 28, Sonnet 1, FS4 | 5 (840), 7 (1631), 9 (3064) |
B. Broadband stimuli and procedure
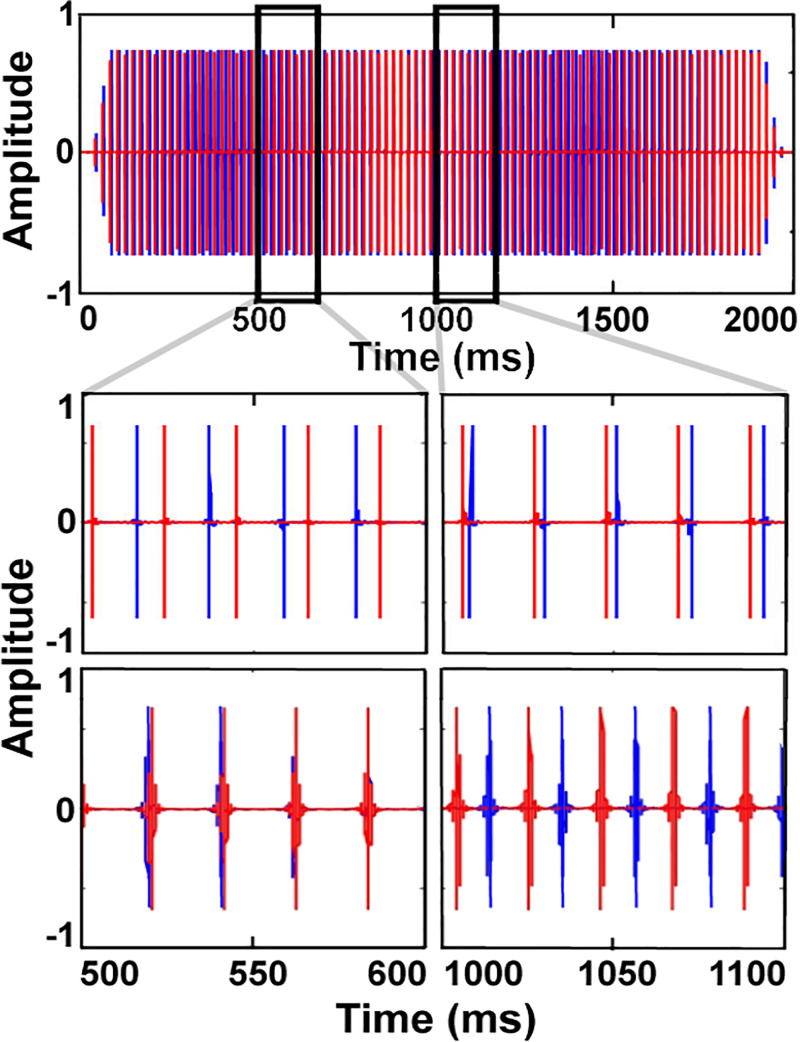
Two sets of broadband acoustic pulse trains were used in a two-interval, two-alternative forced-choice (2I2AFC) task. The nominal pulse rate was roved across intervals between 47 and 53 Hz (selected at random with uniform distribution). Pulses at this rate were presented to one ear (selected at random in each interval); the pulse rate in the other ear was either the same (reference interval) or was higher by 1 Hz (target interval). The different pulse rates between the ears in the target interval led to a dynamic ITD that cycled in and out of phase every 1 s. An illustration of the target interval is shown in Fig. 2. The listeners' task was to select the interval with the dynamic ITD. Feedback was provided after each trial. The dynamic nature of the ITD makes any inherent latency differences between the two ears, and hence any baseline ITD, irrelevant. In both intervals, the starting phase relationship between the pulses in each ear was selected at random with uniform distribution within one cycle of the pulse rate. This randomization eliminated any potential residual effect of baseline differences in latency between the two ears.
FIG. 2.
(Color online.) Illustration of the target stimulus in the temporal-envelope beats task. The full waveform from the target interval in the broadband condition is shown in the first row. The second and third rows provide snapshots of the broadband and narrowband stimuli, respectively. The pulse rate was 1-Hz higher in the right ear (red) than in the left ear (blue), resulting in a dynamic timing relationship between the pulses in the two ears that repeats every second.
All testing took place within a single-walled sound-attenuating booth. Stimuli were generated in matlab (The Mathworks, Natick, MA), converted to an analog signal via an E22 soundcard (Lynx Studio Technology, Costa Mesa, CA) at 24-bit resolution with a sampling rate of 48 kHz, and delivered to the acoustic ear via an insert earphone (3 M E-A-RTONE GOLD 3 A) and to the CI ear via direct audio input. The pulse trains were created by adding together all harmonics of the pulse rate (∼50 Hz) up to 16 kHz in sine phase, with the amplitudes of each harmonic selected to produce a bandpass characteristic with a 4-octave passband from 500 to 8000 Hz and attenuation rates of 48 dB/octave outside the passband. This produced a pulse train with a crest factor of 17.8 dB. The ear with the higher pulse rate was chosen at random on every trial. The pulse trains in each interval were 2 s in total duration, including 100-ms raised-cosine onset and offset ramps, to allow for two full cycles of the 1-Hz beat. A 500-ms silent gap separated the two intervals. The overall root-mean-square (rms) level of each stimulus in the acoustic ear was initially set to 50 dB sound pressure level (SPL), with the exception of S6 who had significant high-frequency hearing loss above 2 kHz, and who required the stimulus level in the acoustic ear to be 60 dB SPL to reach a comfortable loudness. The rms voltage presented to the direct audio input of the CI was approximately 2 mV.
The use of a roved pulse rate (between 47 and 53 Hz), the randomization of the ear receiving the higher pulse rate, and the small (1 Hz) difference in rates between the two ears prevented listeners from performing the task based on monaural cues. Instead, listeners had to be able to make binaural comparisons. A low (∼50 Hz) pulse rate was chosen to ensure that the listeners were sensitive to potential binaural cues in the stimulus; higher pulse rates led to less salient temporal-envelope fluctuations in the acoustic ear (Kohlrausch et al., 2000) and less pulse-rate and ITD sensitivity in the CI ear (Kan and Litovsky, 2015). Finally, the stimuli included relatively long-duration onset and offset ramps to avoid stimulus onset dominance (Rakerd and Hartmann, 1986).
Listeners completed at least 2 blocks of 20 trials each on the broadband task before moving to the narrowband task. Listeners were permitted to move to the narrowband task once they had achieved at least 80% correct on at least one block of the broadband task.
C. Narrowband stimuli and procedure
To measure potential frequency selectivity for binaural temporal-envelope beats, harmonics of the pulse rate up to 16 kHz were again combined, but this time they were attenuated with 48 dB/octave slopes on either side of the center frequency (CF), with no flat passband. An illustration of the narrowband pulse trains appears in the bottom row of Fig. 2. In the acoustic ear, the CF of this triangular filter was equal to one the following values in each trial: 500, 700, 1000, 1400, 2000, 2800, 4000, 5600, or 8000 Hz. In the CI ear, an apical, medial, and basal electrode were tested in each listener by using a filter CF that was equal to the CF of a CI channel allocated to one of these three electrodes along the array. Since the electrodes were not directly stimulated, but were instead stimulated via the CI's sound processor, it is possible that electrodes adjacent to the target electrode were also stimulated at a relatively low current level. Stimuli were otherwise identical to those used in the broadband task (e.g., duration, pulse rates, and rove range).
Within one block, the CI stimulus was fixed and the acoustic-ear stimuli were presented at different CFs several times in random order. Each narrowband stimulus in the acoustic ear was presented at least 90 times for a total of 810 trials per CI stimulus (9 CFs × 90 trials). Occasionally, more blocks (30 trials per CF × 9 CFs = 270 total trials per block) were collected.
Unlike in the broadband task, each acoustic-ear stimulus was balanced in loudness relative to the fixed CI stimulus. This occurred at the beginning of every new session and every time a new CI stimulus was used. On each trial, the CI stimulus was presented first followed after a 20-ms silent gap by the acoustic-ear stimulus. Listeners adjusted the intensity of the acoustic-ear stimulus in three step sizes (5, 2, and 1 dB) without time or repetition limitations until it was judged to be equal in loudness to the CI stimulus. Responses were recorded and used to individually set the levels of the acoustic-ear stimuli. Across listeners and electrodes, this produced an average level at the acoustic ear of 49.9, 53.0, and 53.5 dB SPL at the apical, medial, and basal electrode, respectively.
For loudness-balancing purposes alone, the stimuli had a fixed 50-Hz pulse rate and were 500-ms in duration including 50-ms onset and offset ramps. The level of the narrowband stimulus delivered to the CI ear via direct audio input was fixed at a level of approximately 2 mV, which was reported by all the listeners to be soft but audible. The starting level of the stimulus in the acoustic ear was randomly selected from the range 50 ± 15 dB SPL. Only listener S6 needed levels outside this range to achieve loudness matches at frequencies above 4 kHz, with levels of 66.9 and 76.7 dB SPL at 5600 and 8000 Hz, respectively.
III. RESULTS
All nine listeners were able to achieve at least 80% correct performance on the broadband binaural beats task. Some performed well immediately, whereas others required more practice, but most were able to achieve good performance within 10–20 min.
The results from the narrowband stimuli are shown in Fig. 3 for the individual listeners, with performance plotted as a function of the CF in the acoustic ear.
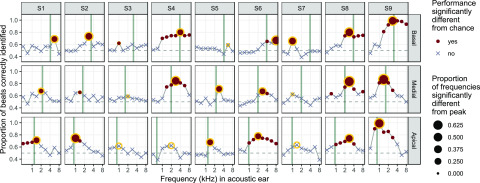
FIG. 3.
(Color online.) Sensitivity to binaural temporal-envelope beats for basal, medial, and apical electrodes (upper, middle, and lower rows, respectively) in nine SSD+CISSD+CISSD+CISSD+CI listeners (columns 1–9). The proportion of trials in which the “beating” interval was correctly identified in a 2I2AFC task is shown as a function of the CF of the stimulus delivered to the acoustic ear. Filled circles indicate conditions where performance was significantly above chance; crosses appear in cases where performance was not significantly different from chance, based on binomial distribution after Bonferroni correction for 9 acoustic frequencies per test electrode; (based on binomial distribution after Bonferroni correction for 9 acoustic frequencies per electrode; α = 0.0056). (top right legend). Circles with a yellow outline denote the acoustic frequency at which best binaural temporal-envelope beat identification (or “peak” performance) was achieved. The diameter of that circle (bottom right legend) indicates the proportion of non-peak comparisons that were significantly different (lower) than peak performance (after Bonferroni correction for 8 comparison points; (based on binomial distribution after Bonferroni correction for 9 acoustic frequencies per electrode; α = 0.00625). The thick vertical line in each panel is located at the CF of the electrode tested in the CI ear. Differences in location between the thick vertical lines and yellow circles indicate a potential tonotopic mismatch between a listener's current clinical map and their acoustic ear.
Across listeners, the mean difference (±1 standard deviation) between the CF assigned to a given electrode and its frequency of best binaural temporal envelope beat sensitivity was −0.15 ± 0.80 octaves when pooled across electrode locations. Omitting non-significant peaks from the analysis resulted in a similar estimate of mean shift (−0.09 ± 0.80 octaves). When assessed separately at the three different locations, the shift was −0.66 ± 0.60 octaves for the apical electrode, −0.05 ± 0.51 octaves for the medial electrode, and +0.27 ± 0.97 octaves for the basal electrode. In all cases, a negative value implies an electrode location that is more basal than it should be, based on its best-matching frequency in the binaural-beats task.
A few interesting patterns emerged at the level of single electrodes. First, some electrodes produced clear sharp peaks in performance at the (presumably) best frequency match in the acoustic ear (e.g., S7, basal electrode), consistent with binaural sensitivity being dependent on a good tonotopic match between the ears, whereas others produced more broadly tuned peaks (e.g., S4, all electrodes), which may be related to spread of excitation (Blanks et al., 2008). Second, some nonmonotonic relationships were observed. For instance, in S3 at the basal electrode, secondary peaks can be observed that appear to be remote from the expected tonotopic location. It may be that such smaller peaks represent cross-turn stimulation (Briaire and Frijns, 2006). Finally, some listeners showed relatively good matches between the assigned frequency and the best match based on binaural-beat detection, as reflected by a match between the vertical gray bar and the yellow circle (e.g., S9, all electrodes), whereas others showed systematic deviations between the two (e.g., S6 and S7).
IV. DISCUSSION
All SSD+CISSD+CI listeners in this study were sensitive to broadband binaural temporal-envelope beats, consistent with previous studies showing ITD sensitivity (Bernstein et al., 2018; Francart et al., 2018). Tonotopic effects with the narrowband stimuli varied between participants and electrodes, but on average the mismatch was greatest (2/3 octave) at the apical electrode and was closer to zero for the medial and basal electrode. Compared with earlier studies using x-ray estimates of electrode position (Landsberger et al., 2015) and ITD discrimination (Bernstein et al., 2018), the mismatch found here was generally in the same direction (with the electrode more basal than indicated by the assigned CF) but tended to be smaller.
Our results extend previous findings by showing that dynamic temporal-envelope ITDs can be used to measure sensitivity to binaural interactions in a way that does not require any knowledge of, or correction for, inherent latency differences between the acoustic- and electric-hearing ear. Finally, it should be noted that the neural mechanisms underlying the SSD+CI patients' detection of the binaural beats is not known; they could be based on the dynamically varying interaural timing cues (McFadden and Pasanen, 1975) or, perhaps more likely, the short-term interaural intensity differences that arise as the stimulus envelopes move in and out of phase (Bernstein and Trahiotis, 1996).
In summary, binaural temporal-envelope beats provide a way to assess binaural interactions without prior knowledge of inherent latency differences between the CI and acoustic ear. This technique could be used to map CIs, with the aim of reducing place of stimulation mismatches between the two ears in SSD+CI listeners. Such remapping, when combined with compensation for latency differences (Seebacher et al., 2019; Wess et al., 2017; Zirn et al., 2015, 2019) could result in improved performance for these patients (Bernstein et al., 2018).
ACKNOWLEDGMENTS
The authors thank Josh Bernstein for helpful discussions and for assistance in piloting the experiment, Douglas Sladen and the Mayo Clinic for assistance in subject recruitment, and Heather Kreft for assistance in collecting pilot data and subject information. This work was supported by the Center for Applied and Translational Sensory Science (CATSS) at the University of Minnesota and by NIH Grant Nos. R01 DC012262 (A.J.O.) and F32 DC016815 (C.E.D).
References
- 1. Bernstein, J. G. W. , Stakhovskaya, O. A. , Schuchman, G. I. , Jensen, K. K. , and Goupell, M. J. (2018). “ Interaural time-difference discrimination as a measure of place of stimulation for cochlear-implant users with single-sided deafness,” Trends Hear. 22, 1–19. 10.1177/2331216518765514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bernstein, L. R. , and Trahiotis, C. (1996). “ Binaural beats at high frequencies: Listeners' use of envelope-based interaural temporal and insensitive disparities,” J. Acoust. Soc. Am. 99, 1670–1679. 10.1121/1.414689 [DOI] [Google Scholar]
- 3. Blanks, D. A. , Buss, E. , Grose, J. H. , Fitzpatrick, D. C. , and Hall, J. W. (2008). “ Interaural time discrimination of envelopes carried on high-frequency tones as a function of level and interaural carrier mismatch,” Ear Hear. 29, 674–683. 10.1097/AUD.0b013e3181775e03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Briaire, J. J. , and Frijns, J. H. M. (2006). “ The consequences of neural degeneration regarding optimal cochlear implant position in scala tympani: A model approach,” Hear. Res. 214, 17–27. 10.1016/j.heares.2006.01.015 [DOI] [PubMed] [Google Scholar]
- 5. Brughera, A. , Dunai, L. , and Hartmann, W. M. (2013). “ Human interaural time difference thresholds for sine tones: The high-frequency limit,” J. Acoust. Soc. Am. 133, 2839–2855. 10.1121/1.4795778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carlyon, R. P. , MacHerey, O. , Frijns, J. H. M. , Axon, P. R. , Kalkman, R. K. , Boyle, P. , Baguley, D. M. , Briggs, J. , Deeks, J. M. , Briarie, J. J. , Barreau, X. , and Dauman, R. (2010). “ Pitch comparisons between electrical stimulation of a cochlear implant and acoustic stimuli presented to a normal-hearing contralateral ear,” J. Assoc. Res. Otolaryngol. 11, 625–640. 10.1007/s10162-010-0222-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dirks, C. , Nelson, P. B. , Sladen, D. P. , and Oxenham, A. J. (2019). “ Mechanisms of localization and speech perception with colocated and spatially separated noise and speech maskers under single-sided deafness with a cochlear implant,” Ear Hear. 40, 1293–1306. 10.1097/AUD.0000000000000708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Francart, T. , Wiebe, K. , and Wesarg, T. (2018). “ Interaural time difference perception with a cochlear implant and a normal ear,” J. Assoc. Res. Otolaryngol. 19, 703–715. 10.1007/s10162-018-00697-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu, H. , and Dietz, M. (2015). “ Comparison of interaural electrode pairing of bilateral cochlear implants,” Trends Hear. 19, 1–22. 10.1177/2331216515617143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kan, A. , and Litovsky, R. Y. (2015). “ Binaural hearing with electrical stimulation,” Hear. Res. 322, 127–137. 10.1016/j.heares.2014.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kohlrausch, A. , Fassel, R. , and Dau, T. (2000). “ The influence of carrier level and frequency on modulation and beat-detection thresholds for sinusoidal carriers,” J. Acoust. Soc. Am. 108, 723–734. 10.1121/1.429605 [DOI] [PubMed] [Google Scholar]
- 12. Landsberger, D. M. , Svrakic, M. , Roland, J. T. , and Svirsky, M. (2015). “ The relationship between insertion angles, default frequency allocations, and spiral ganglion place pitch in cochlear implants,” Ear Hear. 36, e207–e213. 10.1097/AUD.0000000000000163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Licklider, J. C. R. , Webster, J. C. , and Hedlun, J. M. (1950). “ On the frequency limits of binaural beats,” J. Acoust. Soc. Am. 22, 468–473. 10.1121/1.1906629 [DOI] [Google Scholar]
- 14. Litovsky, R. Y. , Moua, K. , Godar, S. , Kan, A. , Misurelli, S. M. , and Lee, D. J. (2019). “ Restoration of spatial hearing in adult cochlear implant users with single-sided deafness,” Hear. Res. 372, 69–79. 10.1016/j.heares.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McFadden, D. , and Pasanen, E. G. (1975). “ Binaural beats at high frequencies,” Science 190, 394–396. 10.1126/science.1179219 [DOI] [PubMed] [Google Scholar]
- 16. Perrott, D. R. , and Musicant, A. D. (1977). “ Rotating tones and binaural beats,” J. Acoust. Soc. Am. 61, 1288–1292. 10.1121/1.381430 [DOI] [PubMed] [Google Scholar]
- 17. Rakerd, B. , and Hartmann, W. M. (1986). “ Localization of sound in rooms III: Onset and duration effects,” J. Acoust. Soc. Am. 80, 1695–1706. 10.1121/1.394282 [DOI] [PubMed] [Google Scholar]
- 18. Reiss, L. A. J. , Ito, R. A. , Eggleston, J. L. , Laio, S. J. , Becker, J. E. , Lakin, C. M. , Warren, F. O. , and McMenomey, S. O. (2015). “ Pitch adaptation patterns in bimodal cochlear implant users: Over time and after experience,” Ear Hear. 36, e23–e34. 10.1097/AUD.0000000000000114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seebacher, J. , Franke-Trieger, A. , Weichbold, V. , Zorowka, P. , and Stephan, K. (2019). “ Improved interaural timing of acoustic nerve stimulation affects sound localization in single-sided deaf cochlear implant users,” Hear. Res. 371, 19–27. 10.1016/j.heares.2018.10.015 [DOI] [PubMed] [Google Scholar]
- 20. Stakhovskaya, O. , Sridhar, D. , Bonham, B. H. , and Leake, P. A. (2007). “ Frequency map for the human cochlear spiral ganglion: Implications for cochlear implants,” J. Assoc. Res. Otolaryngol. 8, 220–233. 10.1007/s10162-007-0076-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wess, J. M. , Brungart, D. S. , and Bernstein, J. G. (2017). “ The effect of interaural mismatches on contralateral unmasking with single-sided vocoders,” Ear Hear. 38, 374–386. 10.1097/AUD.0000000000000374 [DOI] [PubMed] [Google Scholar]
- 22. Williges, B. , Wesarg, T. , Jung, L. , Geven, L. I. , Radeloff, A. , and Jürgens, T. (2019). “ Spatial speech-in-noise performance in bimodal and single-sided deaf cochlear implant users,” Trends Hear. 23, 1–16. 10.1177/2331216519858311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zirn, S. , Angermeier, J. , Arndt, S. , Aschendorff, A. , and Wesarg, T. (2019). “ Reducing the device delay mismatch can improve sound localization in bimodal cochlear implant or hearing-aid users,” Trends Hear. 23, 1–13. 10.1177/2331216519843876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zirn, S. , Arndt, S. , Aschendorff, A. , and Wesarg, T. (2015). “ Interaural stimulation timing in single sided deaf cochlear implant users,” Hear. Res. 328, 148–156. 10.1016/j.heares.2015.08.010 [DOI] [PubMed] [Google Scholar]