Fig. 3.
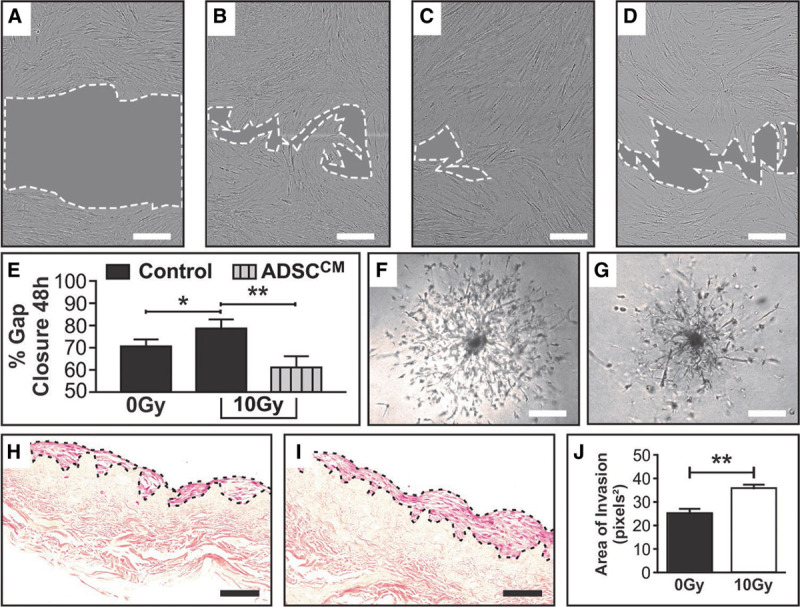
RT x increases the migratory capacity of NHDF, a change that is reversed with the addition of ADSCCM. ×10 objective bright field imaging of NHDF was taken at 0 and 48 h post creation of the scratch wound on a confluent mono-layer of proliferating cells. A, Representative image of NHDF scratch wound area at 0 h, (B) 0Gy NHDF in control media (complete DMEM) scratch wound area at 48 h, (C) 10Gy NHDF in control media (complete DMEM) scratch wound area at 48 h, (D) 10Gy NHDF in ADSCCM scratch wound area at 48 h. (E) Quantification and analyses of the % gap closure at 48 h compared with 0 h controls demonstrating differences resulting from RTx injury and treatment with ADSCCM in a model of fat grafting. % Gap closure between 0Gy (B) and 10Gy (C) NHDF scratch wound areas at the 48 h end-point graphically demonstrates the unexpected increase in migration of 10Gy NHDF, while addition of ADSCCM to 10Gy NHDF (D) mitigated this hypermigratory state. F, 0Gy NHDF cell outgrowth captured at Day 11 demonstrating a symmetrical pattern of invasion migrating from a central core of cells. G, 10Gy NHDF cell outgrowth captured at Day 11 demonstrating asymmetrical and disordered sprouting from a denser central core of cells, captured at ×4 objective using bright field microscopy. H, 0Gy NHDF and (I) 10Gy NHDF on DED matrix (transverse section ×20 objective), with (J) quantification of the NHDF cell layer area demonstrating a significantly thicker layer of cells with a broader front of invasion in the 10Gy irradiated group. In (A–D), scale bar 300μm, dotted white line represents periphery of scratch wound and gray shaded area represents scratch wound area. Scale bars in F–G 300 μm and H–I 50 μm (*P value <0.05; **P value <0.01). Error bars represent SEM, n ≥ 3 for all experiments.
