Abstract
Purpose Olfactory neuroblastoma (ONB) is a rare head and neck cancer believed to be originated from neural crest cells of the olfactory membrane located in the roof of the nasal fossa. This study evaluates clinical outcomes and failure patterns in ONB patients of those patients treated with surgical resection at a high-volume tertiary cancer center.
Methods and Materials Thirty-nine ONB patients who underwent surgical resection at our institution from 1996 to 2017 were retrospectively identified. Univariate, multivariate, and survival analysis were calculated using Cox regression analysis and Kaplan–Meier log-rank.
Results Median follow-up time was 59 months (range: 5.2–236 months). The median overall survival (OS) and disease-free survival (DFS) for the entire cohort were 15 and 7.6 years, respectively. The 5-year cumulative OS and DFS were 83 and 72%, respectively. The 5-year OS for low Hyams grade (LHG) versus high Hyams grade (HHG) was 95 versus 61% ( p = 0.041). LHG was found in 66% of the early Kadish stage patients compared with 28% in the advanced Kadish stage patients ( p = 0.057). On multivariate analysis, HHG and positive node status predicted for worse OS and only HHG predicted for worse DFS. Of note, five patients (all Kadish stage A) who received surgical resection alone had no observed deaths or recurrences with a median follow-up of 44 months (range: 5–235 months).
Conclusion In this retrospective cohort, patients with positive nodes or HHG have significantly worse clinical outcomes. Future studies should explore treatment intensification for HHG or positive nodes.
Keywords: olfactory neuroblastoma, radiation therapy, IMRT
Introduction
Olfactory neuroblastoma (ONB) (previously referred to as esthesioneuroblastoma) is a rare cancer believed to be originated from neural crest cells of the olfactory membrane located in the roof of the nasal fossa. It constitutes ∼3 to 6% of all intranasal cancers. 1 Surveillance, epidemiology, and end results (SEER) data of 311 ONB patients showed the nasal cavity to be the primary site in 75% of cases and that 12.3% presented with nodal involvement. 2 Surgical resection has historically involved craniofacial resection; however, with the advent of endonasal–endoscopic techniques, an endoscopic or combined open approach has been applied to appropriate cases. 3 4 The clinical staging of ONB originated with Kadish et al and dates back to 1976. Early Kadish stage (EKS) includes stage A, disease confined to the nasal cavity; and stage B, disease defined to the nasal cavity and one or more paranasal sinuses; and advanced Kadish stage (AKS) includes stage C, disease extending outside the nasal cavity and paranasal sinuses, including the orbit or intracranial disease; and stage D, nodal disease or distant metastasis. 5 Kadish stage at presentation is 51 and 49% for EKS and AKS, respectively. 6 ONB histological grading is based on the Hyams criteria that grades tumors based on degree of differentiation from low Hyams grade (LHG) (Grade I and II) to high Hyams grade (HHG) (Grade III and IV) based on the tumor architecture, cellular pleomorphism, presence of neurofibrillary matrix and rosettes, mitotic activity, and presence of necrosis or calcifications. 7
Currently, the most accepted treatment for ONB comprises surgical resection with or without adjuvant radiotherapy. Some have suggested that the median survival of patients treated with combined modality is significantly longer to that of patients treated by either surgery or radiotherapy alone. 2 Adjuvant radiation therapy is considered the optimal treatment based on several retrospective reviews showing improved outcomes when using combined surgery and radiation therapy. 8 9 10 11 ONB cancers are sensitive to platinum-based chemotherapies, but the use of chemotherapy is most commonly reserved for locally advanced ONB or metastatic disease. 12
Nonetheless, recurrence rates remain relatively high for ONB. For instance, authors from Mayo Clinic reported a 42% local recurrence rate at 5-year follow-up. 13 14 Furthermore, authors from the University of Virginia analyzed their experience with 40 ONB patients treated with combined surgery and radiation therapy yielding a local and regional failure rates of 55 and 68%, respectively 15 16 To date, there has only been one prospective trial from Massachusetts General Hospital. They reported a 5-year survival rate of 74% with the use of neoadjuvant cisplatin and etoposide followed by proton–photon radiotherapy to 69.2 cobalt-Gray equivalents (CGE) using 1.6 to 1.8 CGE per fraction twice daily. 17
While the optimal management of ONB remains undefined, its small numbers preclude clarification through prospective trials. Several key questions remain including the exact cell of origin, molecular subtypes, optimal radiation targets and dosing, and which systemic therapies are best suited for advanced or metastatic disease.
Methods and Materials
Patient Data
As a part of an Institutional Review Board-approved study, a total of 39 patients with histologically confirmed diagnosis of ONB, treated with surgical resection with or without adjuvant radiation therapy from 1996 to 2017, were retrospectively identified. Medical records of 39 patients were reviewed for clinical and pathological characteristics. To avoid including lesions that are currently classified as tumors other than ONB, all specimens were reviewed by a senior head and neck pathologist, who also updated the Hyams grading (changed in a total of seven patients). Radiation and chemotherapy records were all reviewed. Disease staging was determined from medical record or imaging studies using the modified Kadish system. 5
Statistical Analysis
Chi-squared and Fisher's exact tests were used to compare variables and t -test was used to compare continuous variables as appropriate in the study. The Kaplan–Meier method was utilized to analyze overall survival (OS), disease-free survival (DFS), locoregional control, and distant metastasis free survival. The log-rank test was used to compare survival curves between groups. DFS was defined as number of patients alive without evidence of disease. The impact of covariates was examined using multivariate Cox proportional hazard regression analysis. All tests were two-sided, and a p -value of < 0.05 was considered significant in this study. All statistical analysis was performed using the IBM SPSS V25.0 software package.
Radiation Treatment Overview
Radiation therapy was delivered to patients in the adjuvant setting for 34 (87%) patients. Intraoperative radiation therapy (IORT) followed by external beam radiation therapy (EBRT) was delivered to 11 (28%) patients. The median total radiation dose delivered for all patients who received EBRT without IORT was 6,000 cGy (range: 5,400–6,600 cGy). Early in the study time frame from 1996 to 2009, the radiation modality used was three-dimensional (3D) conformal radiation therapy. From 2010 to 2017, patients were treated with intensity-modulated radiation therapy (IMRT), and in 2016 volumetric modulated arc therapy (VMAT) was utilized. Three patients transferred care to an outside institution to receive proton therapy. Chemotherapy was administered at any point of the treatment course for six (15%) patients. Three patients were administered induction chemotherapy consisting of cisplatin and etoposide.
Surgical Techniques
From the years 1996 to 2008, the surgical technique utilized in 15 patients (38.5%) was typically a sublabial degloving or lateral rhinotomy approach with bifrontal craniotomy with or without intraoperative high-dose radiotherapy (IO-HDR). Reconstruction at that time typically consisted of a pericranial flap with bone graft and intranasal Foley, split-thickness skin graft, and Vaseline gauze packing. Starting in 2009 with the arrival of endoscopic endonasal approach trained faculty, 24 patients (61.5%) underwent an endoscopic endonasal anterior craniofacial approach with adjuvant IMRT or VMAT. Reconstruction is often with a pericranial flap reinforced with fascia lata, fat graft, or nasal flaps (when available). The 5-year OS and DFS were greater in the endoscopic approach compared with craniotomy approach, but this did not reach statistical significance, 87.9 and 79.5% versus 69.8 and 58.8% ( p = 0.38).
Intraoperative Radiation Therapy
The use of IORT was administered in 11 (28%) of all surgical cases. IO-HDR was utilized within a sponge applicator. Three to five catheters were typically utilized depending on the surgical cavity size and placed 1 cm apart within a foam applicator. The patients remained under monitoring, while a dose of 1,000 to 1,500 cGy was delivered to a 0.5 cm depth to the treatment volume within the base of the skull.
Results
Patient and Clinical Characteristics
Basic demographic and clinical data for the 39 patients are outlined in Table 1 . Our cohort comprised 14 women (36%) and had a mean age of 50 years (range: 19–78 years). Hyams grading was reviewed by a head and neck pathologist, yielding a final Hyams low grade in 22 (56%) and a high grade in 17 (44%) patients. Hyams grade was changed in seven patients from the original pathology report. A total of 10 patients (44%) were EKS and 22 (56%) were AKS. The number of patients with pathologically confirmed nodes at the time of surgery was 8 (21%).
Table 1. Patient characteristics ( n = 39) .
| Number | % or Mean | Min | Max | ||
|---|---|---|---|---|---|
| Sex | |||||
| Female | 14 | 36 | |||
| Male | 25 | 64 | |||
| Age at diagnosis | 50 | 19 | 78 | ||
| Age | |||||
| <50 | 15 | 41 | |||
| ≥50 | 22 | 59 | |||
| Hyams grade | |||||
| Low | 22 | 56 | |||
| High | 17 | 44 | |||
| Kadish stage | |||||
| A | 5 | 13 | |||
| B | 5 | 13 | |||
| C | 22 | 56 | |||
| D | 7 | 18 | |||
| Node | |||||
| Negative | 31 | 79 | |||
| Positive | 8 | 21 | |||
| Radiation | |||||
| None | 5 | 13 | |||
| Yes | 34 | 87 | |||
| Radiation dose | 60 | 50.4 | 69 | ||
| Radiation type | |||||
| None | 5 | 13 | |||
| IORT + adjuvant EBRT | 11 | 28 | |||
| adjuvant EBRT | 23 | 59 | |||
| Radiation dose | |||||
| None | 5 | 13 | |||
| <60 | 10 | 25 | |||
| >60 | 24 | 61 | |||
| Chemotherapy | |||||
| Yes | 6 | 15 | |||
| None | 33 | 85 |
Abbreviations: EBRT, external beam radiation therapy; IORT, intraoperative radiation therapy.
Clinical Outcomes
With a median follow-up of 59 months, the 5- and 10-year OS was 95 and 61% in the LHG patients, respectively, compared with 61 and 32% in the HHG patients ( p = 0.041, hazard ratio [HR]: 2.9, 95% confidence interval [CI]: 1.18–10.1) ( Fig. 1A ). The 5- and 10-year OS was 100 and 83% in the EKS patients, respectively, compared with 73 and 38% in the AKS patients ( p = 0.078, HR: 4.0, 95% CI: 1.62–38.1) ( Fig. 1B ). The 5-year OS/DFS was 100%/100%, 100%/66%, 100%/75% and 59%/47% for EKS–LHG, EKS–HHG, AKS–LHG, and AKS–HHG, respectively. Five patients in the EKS group received surgical resection alone, and there were no observed deaths or recurrences with a median follow-up of 44 months (range: 5–235 months) ( Table 2 ). A total of 31 (79%) patients in the entire cohort presented with pathologically or clinically node-negative disease. Of the node-negative patients, 13 (41%) received elective nodal radiation (ENI) (12/13 of these patients had Kadish Stage C) ( Fig. 2 ). The 5-year OS was 90% for node-negative patients versus 46% for node-positive patients ( p < 0.001, HR: 11.7, 95% CI: 2.42–55.3). There were seven nodal recurrences in the entire cohort, with one nodal recurrence 16 years following treatment (median: 59 months, range: 6–165 months). In node-negative patients, the 10-year nodal-failure rate was 54% in patients treated with primary site radiation alone compared with 0% with the addition of ENI ( p = 0.22) ( Fig. 2 ).
Fig. 1.
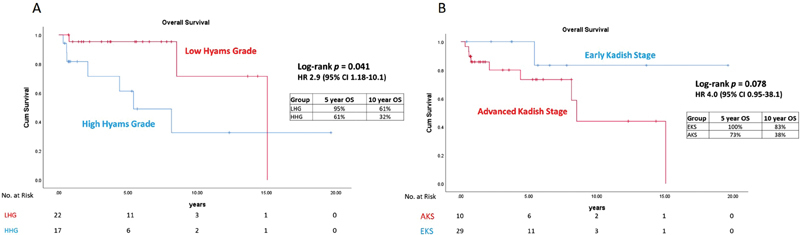
Kaplan–Meier curves comparing overall survival for ( A ) low Hyams grade (LHG) versus high Hyams grade (HHG) and ( B ) advanced Kadish stage (AKS) versus early Kadish stage (EKS). CI, confidence interval; HR, hazard ratio; OS, overall survival.
Table 2. Recurrence location ( n = 39) .
| Count | % | |
|---|---|---|
| Local recurrence, total patients | 8 | 21 |
| Neck recurrence | 7 | 18 |
| Level I | 1 | 14 |
| Level II only | 2 | 29 |
| Level II + III | 3 | 42 |
| Level IV | 1 | 14 |
| Level V | 0 | 0 |
| Distant recurrence | 7 | 18 |
| Brain | 2 | 28 |
| Spine | 4 | 57 |
| Liver | 1 | 14 |
| Overall recurrence | 13 | 33 |
Fig. 2.
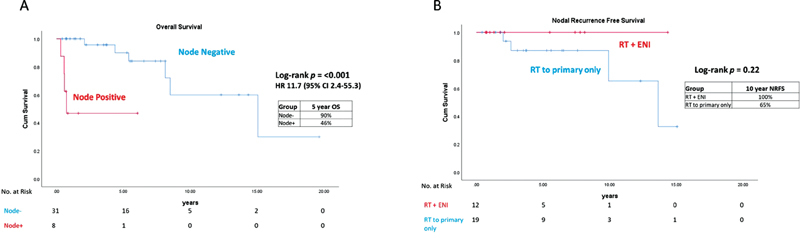
Kaplan–Meier curves comparing ( A ) overall survival for node positive versus node negative patients and ( B ) nodal recurrence free survival for patients who received ENI versus primary site alone radiation. CI, confidence interval; ENI, elective nodal radiation; HR, hazard ratio; NRFS, nodal recurrence free survival; OS, overall survival; RT, radiation therapy.
Patterns of Failure
There was a total of 13 recurrences (33.3%) in this patient cohort. There were eight local recurrences (21%), seven nodal recurrences (18%), and seven distant recurrences (18%) ( Table 2 ). The most common site of neck recurrence was level II (85%) followed by level III (42%). For both level I and IV, there was one recurrence and zero recurrences in level V. The most common site for distant failure was the spine (57%). Surgery was the most common salvage therapy; eight patients (61.5%) were treated with salvage surgery. Salvage radiation therapy was used in five recurrences (38%), and three (23%) patients were treated with salvage chemotherapy.
Multivariate Analysis
Cox regression univariate and multivariate analysis was performed for OS and disease recurrence for the clinical and treatment variables. On multivariate Cox proportional hazards regression analysis, only HHG (HR: 22.1, 95% CI: 2.2–218) and positive lymph nodes (HR: 14.7, 95% CI: 1.9–111) were associated with worse OS ( Table 3 ). On multivariate Cox regression analysis, only HHG (HR: 17.0, 95% CI: 1.01–288.4) was associated with worse DFS ( Table 3 ).
Table 3. Univariate and multivariate Cox regression analysis for OS.
| Univariate | Multivariate | |||
| Variable | HR (95% CI) | p -Value | HR (95% CI) | p -Value |
| Age at diagnosis | 0.99 (0.94–1.03) | 0.69 | ||
| Sex | 1.94 (0.55–6.77) | 0.29 | ||
| Hyams grade, high vs. low | 3.75 (0.96–14.6) | 0.05 | 22.1 (2.2–218) | 0.008 |
| Kadish stage, A-B vs. C-D | 0.22 (0.02–1.80) | 0.16 | ||
| Node status, positive vs. negative | 11.7 (2.42–55.5) | 0.002 | 14.7 (1.9–111) | 0.01 |
| Chemotherapy, yes vs. no | 12.1 (1.87–76.9) | 0.009 | 0.41 (0.04–3.87) | 0.43 |
| Radiation, yes vs. no | .03 (0.0–48.7) | 0.35 | ||
| Radiation, IORT vs. EBRT | .85 (0.18–3.9) | 0.83 | ||
| Radiation, ENI vs. primary only | .686 (0.2–4.3) | 0.99 | ||
| Surgery type, craniotomy vs. endoscopic | 0.56 (0.12–2.59) | 0.46 | ||
| Diagnosis era, 1996–2010 vs. 2010–2017 | 0.94 (0.20–4.37) | 0.94 | ||
| Univariate and multivariate Cox regression of disease recurrence score | ||||
| Univariate | Multivariate | |||
| Variable | HR (95% CI) | p | HR (95% CI) | p -Value |
| Age at diagnosis | 0.349 (0.07–1.6) | 0.18 | ||
| Sex | 1.05 (0.32–3.48) | 0.92 | ||
| Hyams grade, high vs. low | 12.5 (1.55–49.3) | 0.01 | 17.0 (1.01–288.4) | 0.049 |
| Kadish stage, A-B vs. C-D | 0.75 (0.23–2.5) | 0.64 | ||
| Node status, positive vs. negative | 6.06 (1.33–27.7) | 0.02 | 8.3 (0.57–10.5) | 0.16 |
| Chemotherapy, yes vs. no | 0.44 (0.04–4.46) | 0.48 | ||
| Radiation, yes vs. no | 0.37 (0.04–3.0) | 0.36 | ||
| Radiation, IORT vs. EBRT | 1.6 (0.44–6.0) | 0.46 | ||
| Radiation, ENI vs. primary only | 0.42 (0.04–3.9) | 0.45 | ||
| Surgery type, craniotomy vs. endoscopic | 0.71 (0.19–2.6) | 0.6 | ||
| Diagnosis era, 1996–2010 vs. 2010–2017 | 1.8 (0.36–9.4) | 0.44 | ||
Abbreviations: CI, confidence interval; EBRT, external beam radiation therapy; ENI, elective nodal radiation; HR, hazard ratio; IORT, intraoperative radiation therapy; OS, overall survival.
Discussion
ONB is rare and recurrences can occur several years after treatment so initial treatments mostly appear successful unless long-term follow-up is maintained. Surgery is the mainstay of treatment for early and late stage ONB. Radiation alone could be considered for select Kadish A patients but should not be recommended in Kadish B or higher since local control rates were shown to be only 58 and 18.9%, for Kadish B and C, respectively. 18 SEER analysis of 311 ONB patients showed an improvement in OS with combined radiation and surgery. 2 Other factors associated with improved survival were Kadish stage, age, and lymph node status. These factors also are predictors of OS in the patient cohort used for this study.
Surgery has evolved over the years from a craniofacial resection to a less invasive approaches such as endonasal endoscopic resection. A meta-analysis of 379 patients with ONB showed improved OS in patients who underwent endoscopic resection compared with open surgery. 19 Whether endoscopic resection for more advanced disease is equivalent to open surgery remains unknown. The typical surgical approach utilized at our institution is an endoscopic endonasal resection with bicoronal incision for pericranial flap harvest. Neck dissection is performed in the setting of node positive disease. One question in the field is when a unilateral resection is appropriate for smell preservation. 20 Due to recent anatomic analysis of the course of olfactory filaments, we recommend avoiding unilateral resection if disease extends up to the nasal septum. 21
Several prior studies have examined the role of radiation in ONB. The challenge of treating this cancer lies in the location of the primary tumor close to critical base of skull structures. For our institution, the early approach to reduce radiation toxicities was to deliver IORT followed by reduced external beam doses. With the introduction of endoscopic surgeries and IMRT, external beam radiation could be safely delivered to higher doses. There were no differences in either survival or recurrence rates using either the IORT followed by EBRT approach or surgery and adjuvant IMRT. The IORT was delivered using HDR brachytherapy using catheters with intraoperative doses of 1,000 to 1,500 cGy followed by 3D conformal radiation to doses of 4,500 to 5,000 cGy. The median EBRT dose using IMRT was 6,000 cGy (5,400–6,600 cGy). Another approach to minimize dose to critical structures is using proton therapy. The advantage of proton therapy is the ability to deliver maximal energy to a defined range. Three of our patients were sent to outside facility to receive proton therapy. Nishimura et al reported their experience using proton therapy to ONB Kadish B or C tumors to a dose of 6500 cGy in 26 fractions. The 5-year OS was 94% and local progression free survival was 84% without late Grade III toxicities. 22
For node negative patients, ENI is recommended for patients with Kadish C or D and can be omitted for earlier stage based on several retrospective studies that showed a nodal failure rate of 27 to 33% for patients who did not receive ENI. 23 24 Similarly, herein we report a 10-year nodal failure rate of 27% for patients with node negative disease not treated with ENI. A MD Anderson retrospective review of a cohort of 71 patients investigated the role of ENI and showed a total of 22 (31%) patients received ENI, and utilization of ENI was associated with improved regional nodal control at 5 years (100 vs. 82% p < 0.01). There was no difference in OS or DFS among patients receiving ENI versus no ENI. 23 In this study, the most common neck recurrence was in level II with one recurrence at level I and IV and no recurrences in level V. Currently for adjuvant radiation therapy, we treat the postoperative cavity and positive lymph nodes to a dose of 60 to 66 Gy, and ENI, covering the bilateral levels I through IV cervical neck and retropharyngeal lymph nodes (we consider treating level V especially if there is extension into the nasopharynx) to a dose between 54 and 59.4 Gy.
In our cohort, we found OS in the LHG patients was nearly double in time compared with HHG. As of now there are no specific recommendations for treatment escalation in HHG. Chemotherapy at our institution was rarely used in the definitive setting for this cohort of patients. The use of chemotherapy has increasingly been adopted for Kadish stage C patients. 8 25 Neoadjuvant chemotherapy has been reported to have excellent outcomes in smaller retrospective studies 16 26 Polin et al reviewed 34 patients treated with neoadjuvant radiation with or without chemotherapy. Two-thirds of the patients treated with neoadjuvant therapy showed significant reduction in tumor burden, and response to neoadjuvant therapy predicted for lower rates of disease-related mortality. 27 In another institutional review, 11 ONB patients were treated with etoposide (75 mg/m 2 ), ifosfamide (1000 mg/m2), and cisplatin (20 mg/m2) all administered intravenously on days 1 to 5. A total of 9 patients achieved objective responses, and two were found to have complete responses. 28 Considering that ⅔ of the recurrences are either local or regional, escalation of adjuvant treatment for Kadish C patients with CRT is being considered at our institution.
We report here a high-volume head and neck tertiary cancer institute's experience in the treatment and outcomes for this rare disease with robust median long-term follow-up of 5 years. This study has similar OS rates compared with the other published series for this rare disease ( Table 4 ). Future directions for the management of ONB will need to address optimal treatments for recurrences (e.g., leptomeningeal spread and systemic dissemination of disease). One potential treatment that could be explored is somatostatin analogues. Czapiewski et al evaluated the cellular expression of somatostatin receptors (SSTR) on a cohort of 40 ONBs and found 75% were positive for SSTR2A and 7.5% positive for SSTR5. 29 Other molecular targets that have been studied include inhibitors of platelet-derived growth factor (i.e., sunitinib) and sonic hedgehog pathway including Patched1, Gli1, and Gli2. 30 31
Table 4. Studies of patient numbers and clinical outcomes for ONB published from 1970 to 2017.
| First author | Institution | Sample size | Median follow-up months | Treatment summary | RT dose mean (Gy) | 5-year OS | 5-year LRC | Study type | Years |
|---|---|---|---|---|---|---|---|---|---|
| Fitzek 17 | Harvard | 19 (9 ONB) | 45 | Cisplatin + etoposide 2 cycles followed by proton-photon RT to 69.2 cobalt-Gray (CGE) BID | 69.2 | 74% | 88% | Prospective | 1992–1998 |
| Dulguerov 11 | UCLA | 24 | Not stated | Surgery alone (29%), RT alone (21%), surgery + RT (50%) | 60 | 74% | 67% | Retrospective | 1970–1990 |
| Resto 10 | Johns Hopkins | 20 | 67.2 | Surgery + RT (75%) | 64.8 | 80% | 50–90% | Retrospective | 1981–1998 |
| Diaz 8 | MDA | 30 | 87 | Surgery + RT (76%) | 59.4 | 89% | 69% | Retrospective | 1979–2002 |
| Loy 16 | Virginia | 50 | 93 | Kadish A or B received preoperative RT followed by craniofacial resection. Kadish C received preoperative sequential chemotherapy + RT followed by resection | 50 | 87% | 66% | Retrospective | 1976–2004 |
| Van Gompel 14 | Mayo Clinic | 109 | 61 | Surgery + RT (84%) | 54.6 | 63% | Not Stated | Retrospective | 1962–2009 |
| Wolfe | Ohio State | 39 | 59 | Surgery + RT (88%) | 60 | 83% | 79% | Retrospective | 1996–2017 |
Abbreviations: LRC, locoregional control; ONB, olfactory neuroblastoma; OS, overall survival. RT, radiation therapy.
The results of this study should be interpreted within the context of its limitations, foremost among them being the use of a single-institution, retrospective dataset for patients for whom treatment decisions were not randomly made. In addition, patients in this study were treated over a 30-year period; so clinical outcomes could be impacted by higher quality imaging, radiation planning, surgical techniques and postoperative care, systemic agents, etc. Although adjuvant radiation is the standard of care, five patients with EKS and LHG in this cohort were treated with surgical resection alone and none of these patients had recurrences up to 5 years post-surgery. For patients with HHG or AKS, treatment escalation with either neoadjuvant chemotherapy or concurrent chemoradiation should be considered. Based on our institutional experience, node-negative patients may also benefit of elective nodal irradiation, although validation in a larger cohort is warranted.
Conflict of Interest None declared.
Co-first authors, contributed equally.
Erratum: This article was updated as per Erratum published on July 22, 2019. (DOI: 10.1055/s-0039-1694000). The name of Dr. Mauricio Games has now been added to the author byline.
References
- 1.Goldsweig H G, Sundaresan N. Chemotherapy of recurrent esthesioneuroblastoma. Case report and review of the literature. Am J Clin Oncol. 1990;13(02):139–143. doi: 10.1097/00000421-199004000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Jethanamest D, Morris L G, Sikora A G, Kutler D I. Esthesioneuroblastoma: a population-based analysis of survival and prognostic factors. Arch Otolaryngol Head Neck Surg. 2007;133(03):276–280. doi: 10.1001/archotol.133.3.276. [DOI] [PubMed] [Google Scholar]
- 3.Gallia G L, Asemota A O, Blitz A Met al. Endonasal endoscopic resection of olfactory neuroblastoma: an 11-year experience J Neurosurg 2018;•••:1–7. [DOI] [PubMed] [Google Scholar]
- 4.Harvey R J, Nalavenkata S, Sacks R et al. Survival outcomes for stage-matched endoscopic and open resection of olfactory neuroblastoma. Head Neck. 2017;39(12):2425–2432. doi: 10.1002/hed.24912. [DOI] [PubMed] [Google Scholar]
- 5.Kadish S, Goodman M, Wang C C. Olfactory neuroblastoma. A clinical analysis of 17 cases. Cancer. 1976;37(03):1571–1576. doi: 10.1002/1097-0142(197603)37:3<1571::aid-cncr2820370347>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 6.Broich G, Pagliari A, Ottaviani F.Esthesioneuroblastoma: a general review of the cases published since the discovery of the tumour in 1924 Anticancer Res 199717(4A):2683–2706. [PubMed] [Google Scholar]
- 7.Thompson L DR. Olfactory neuroblastoma. Head Neck Pathol. 2009;3(03):252–259. doi: 10.1007/s12105-009-0125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz E M, Jr, Johnigan R H, III, Pero C et al. Olfactory neuroblastoma: the 22-year experience at one comprehensive cancer center. Head Neck. 2005;27(02):138–149. doi: 10.1002/hed.20127. [DOI] [PubMed] [Google Scholar]
- 9.Chao K S, Kaplan C, Simpson J R et al. Esthesioneuroblastoma: the impact of treatment modality. Head Neck. 2001;23(09):749–757. doi: 10.1002/hed.1107. [DOI] [PubMed] [Google Scholar]
- 10.Resto V A, Eisele D W, Forastiere A, Zahurak M, Lee D J, Westra W H. Esthesioneuroblastoma: the Johns Hopkins experience. Head Neck. 2000;22(06):550–558. doi: 10.1002/1097-0347(200009)22:6<550::aid-hed2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Dulguerov P, Calcaterra T. Esthesioneuroblastoma: the UCLA experience 1970-1990. Laryngoscope. 1992;102(08):843–849. doi: 10.1288/00005537-199208000-00001. [DOI] [PubMed] [Google Scholar]
- 12.McElroy E A, Jr, Buckner J C, Lewis J E.Chemotherapy for advanced esthesioneuroblastoma: the Mayo Clinic experience Neurosurgery 199842051023–1027., discussion 1027–1028 [DOI] [PubMed] [Google Scholar]
- 13.Morita A, Ebersold M J, Olsen K D, Foote R L, Lewis J E, Quast L M.Esthesioneuroblastoma: prognosis and management Neurosurgery 19933205706–714., discussion 714–715 [DOI] [PubMed] [Google Scholar]
- 14.Van Gompel J J, Giannini C, Olsen K D et al. Long-term outcome of esthesioneuroblastoma: Hyams grade predicts patient survival. J Neurol Surg B Skull Base. 2012;73(05):331–336. doi: 10.1055/s-0032-1321512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eden B V, Debo R F, Larner J M et al. Esthesioneuroblastoma. Long-term outcome and patterns of failure--the University of Virginia experience. Cancer. 1994;73(10):2556–2562. doi: 10.1002/1097-0142(19940515)73:10<2556::aid-cncr2820731017>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 16.Loy A H, Reibel J F, Read P W et al. Esthesioneuroblastoma: continued follow-up of a single institution's experience. Arch Otolaryngol Head Neck Surg. 2006;132(02):134–138. doi: 10.1001/archotol.132.2.134. [DOI] [PubMed] [Google Scholar]
- 17.Fitzek M M, Thornton A F, Varvares M et al. Neuroendocrine tumors of the sinonasal tract. Results of a prospective study incorporating chemotherapy, surgery, and combined proton-photon radiotherapy. Cancer. 2002;94(10):2623–2634. doi: 10.1002/cncr.10537. [DOI] [PubMed] [Google Scholar]
- 18.Benfari G, Fusconi M, Ciofalo A et al. Radiotherapy alone for local tumour control in esthesioneuroblastoma. Acta Otorhinolaryngol Ital. 2008;28(06):292–297. [PMC free article] [PubMed] [Google Scholar]
- 19.Devaiah A K, Andreoli M T. Treatment of esthesioneuroblastoma: a 16-year meta-analysis of 361 patients. Laryngoscope. 2009;119(07):1412–1416. doi: 10.1002/lary.20280. [DOI] [PubMed] [Google Scholar]
- 20.Tajudeen B A, Adappa N D, Kuan E C et al. Smell preservation following endoscopic unilateral resection of esthesioneuroblastoma: a multi-institutional experience. Int Forum Allergy Rhinol. 2016;6(10):1047–1050. doi: 10.1002/alr.21794. [DOI] [PubMed] [Google Scholar]
- 21.Gomez Galarce M, Yanez-Siller J C, Carrau R L et al. Endonasal anatomy of the olfactory neural network: Surgical implications. Laryngoscope. 2018;128(11):2473–2477. doi: 10.1002/lary.27194. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura H, Ogino T, Kawashima M et al. Proton-beam therapy for olfactory neuroblastoma. Int J Radiat Oncol Biol Phys. 2007;68(03):758–762. doi: 10.1016/j.ijrobp.2006.12.071. [DOI] [PubMed] [Google Scholar]
- 23.Jiang W, Mohamed A SR, Fuller C D et al. The role of elective nodal irradiation for esthesioneuroblastoma patients with clinically negative neck. Pract Radiat Oncol. 2016;6(04):241–247. doi: 10.1016/j.prro.2015.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noh O K, Lee S W, Yoon S M et al. Radiotherapy for esthesioneuroblastoma: is elective nodal irradiation warranted in the multimodality treatment approach? Int J Radiat Oncol Biol Phys. 2011;79(02):443–449. doi: 10.1016/j.ijrobp.2009.10.067. [DOI] [PubMed] [Google Scholar]
- 25.McLean J N, Nunley S R, Klass C, Moore C, Müller S, Johnstone P A. Combined modality therapy of esthesioneuroblastoma. Otolaryngol Head Neck Surg. 2007;136(06):998–1002. doi: 10.1016/j.otohns.2006.11.051. [DOI] [PubMed] [Google Scholar]
- 26.Turri-Zanoni M, Maragliano R, Battaglia P et al. The clinicopathological spectrum of olfactory neuroblastoma and sinonasal neuroendocrine neoplasms: Refinements in diagnostic criteria and impact of multimodal treatments on survival. Oral Oncol. 2017;74:21–29. doi: 10.1016/j.oraloncology.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Polin R S, Sheehan J P, Chenelle A G et al. The role of preoperative adjuvant treatment in the management of esthesioneuroblastoma: the University of Virginia experience. Neurosurgery. 1998;42(05):1029–1037. doi: 10.1097/00006123-199805000-00045. [DOI] [PubMed] [Google Scholar]
- 28.Kim D-W, Jo Y-H, Kim J H et al. Neoadjuvant etoposide, ifosfamide, and cisplatin for the treatment of olfactory neuroblastoma. Cancer. 2004;101(10):2257–2260. doi: 10.1002/cncr.20648. [DOI] [PubMed] [Google Scholar]
- 29.Czapiewski P, Kunc M, Gorczyński A et al. Frequent expression of somatostatin receptor 2a in olfactory neuroblastomas: a new and distinctive feature. Hum Pathol. 2018;79:144–150. doi: 10.1016/j.humpath.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Mao L, Xia Y P, Zhou Y Net al. Activation of sonic hedgehog signaling pathway in olfactory neuroblastoma Oncology 200977(3-4):231–243. [DOI] [PubMed] [Google Scholar]
- 31.Preusser M, Hutterer M, Sohm M et al. Disease stabilization of progressive olfactory neuroblastoma (esthesioneuroblastoma) under treatment with sunitinib mesylate. J Neurooncol. 2010;97(02):305–308. doi: 10.1007/s11060-009-0027-x. [DOI] [PubMed] [Google Scholar]


