Figure 3: Cellular disease mechanisms associated with NCT defects in ALS/FTD.
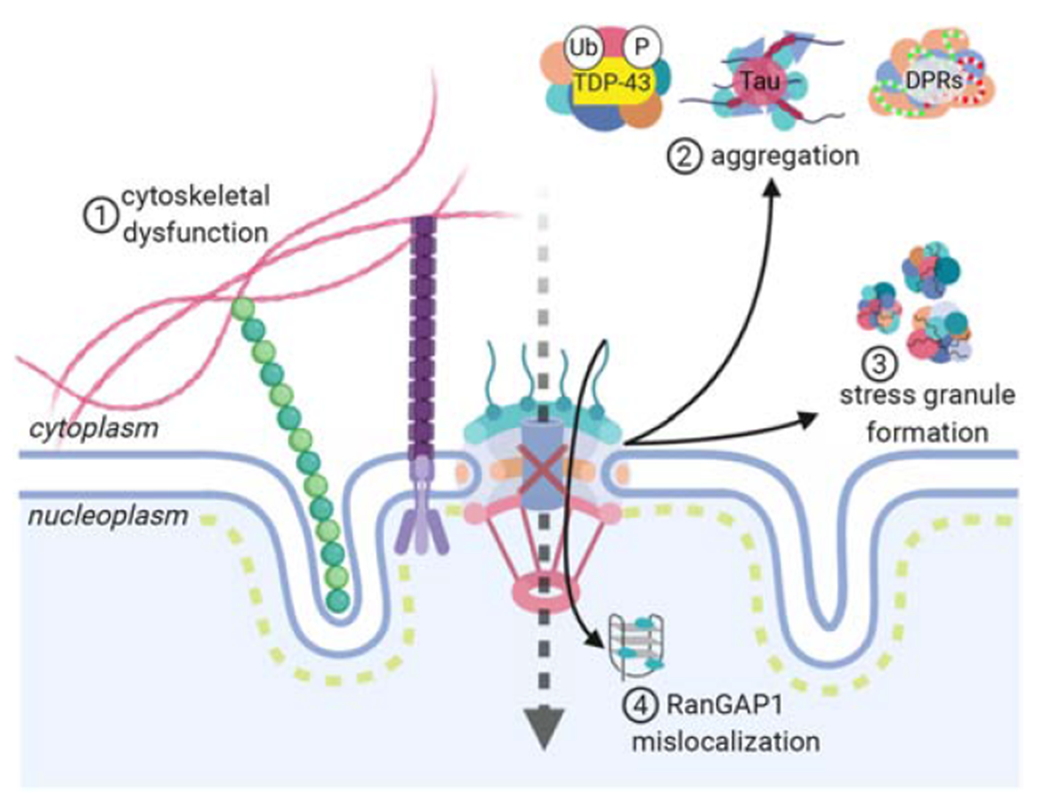
Cytoskeletal dysfunction can lead to an increase in the force exerted on the nucleus, causing the formation of nuclear invaginations and disrupting the integrity of the nuclear lamina (1). Abnormal protein aggregation (2) and stress granule formation (3) sequester nucleoporins and transport proteins in the cytoplasm, affecting the integrity of the nuclear pore and impairing nuclear import. C9-ALS-associated HREs bind and sequester RanGAP1 in the nucleus, thereby disrupting the nuclear gradient of Ran and leading to NCT defects (4).
