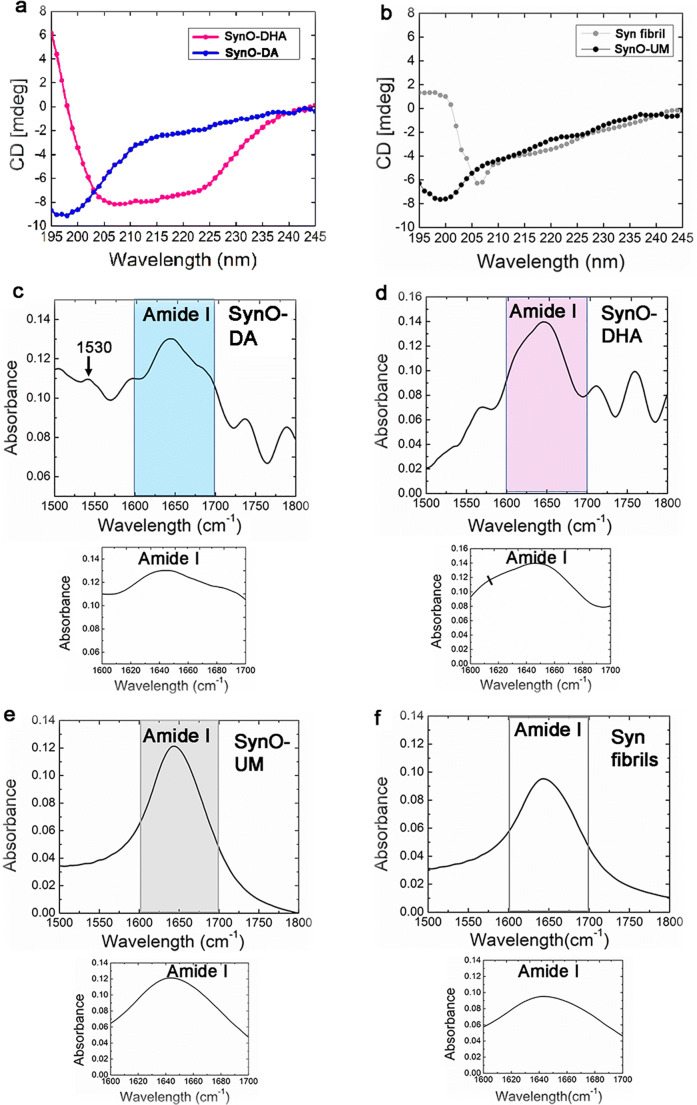
Fig. 3.
Biophysical characterization of DA- and DHA-modified α-Syn oligomeric polymorphs. a CD spectra of SynO-DA and SynO-DHA. SynO-DA shows a minimum around 195 nm indicating mostly random coil, whereas SynO-DHA shows two minima at 208 and 222 nm, suggesting α-helical structure. b CD spectra of SynO-UM show random coil and β-sheet, whereas, Syn fibrils showed α-helical and β-sheet structures. c FTIR spectrum of SynO-DA with the inset showing 1600 to 1700 cm−1 corresponding to the amide I region. This oligomer preparation mostly contains random coil with a peak around 1648–1650 cm−1. A small absorption peak at 1530 cm−1 in amide II region corresponds to β-sheet structure (marked by black arrow). d FTIR spectrum of SynO-DHA shows α-helical structure with an absorption at 1652 cm−1. The spectrum also indicates a cross-β-structure (1614 cm−1), marked by a black line in the inset. e, f FTIR absorption spectrum of SynO-UM mostly shows random coil, while an enlarged peak around 1630–1656 cm−1 was observed for Syn fibrils, indicative of β-sheet and α-helical structures