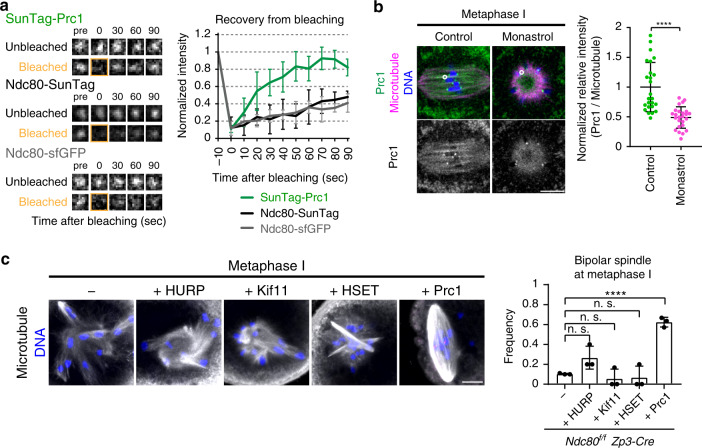
Fig. 5. Ndc80/Nuf2 concentrates dynamic Prc1 at kinetochores for spindle bipolarization.
a Dynamic exchange of Prc1 at kinetochores. In oocytes at prometaphase I (1.5 h after NEBD), SunTag-Prc1 (24xGCN4-Prc1 coexpressed with scFv-sfGFP, green) signals at kinetochores were bleached, and the recovery was monitored (n = 5, 5, 5 kinetochores). Note that the recovery curve of SunTag-Prc1 signals indicates the turnover of Prc1 at kinetochores rather than the turnover of scFv on 24xGCN4 in this time range, which was confirmed by Ndc80-SunTag (Ndc80-24xGCN4 coexpressed with scFv-sfGFP) exhibiting similar recovery curves to Ndc80-sfGFP. See also Supplementary Movie 7. b Kif11-dependent Prc1 enrichment along kinetochore-proximal microtubules of the bipolar spindle. Control or monastrol-treated oocytes at metaphase I (4–6 h after NEBD) were stained for Prc1 (green), microtubules (magenta), and DNA (Hoechst33342, blue). The oocytes were treated with a cold buffer for 1 min before fixation to facilitate antibody penetration into the spindle. Prc1 signals along kinetochore-proximal microtubule bundles were measured, and their ratio to microtubule signals was calculated (n = 25, 25 locations from 5, 5 oocytes. Three independent experiments were performed). ****p < 0.0001 (p = 8.1E−07) by two-tailed unpaired Student’s t-test. c Prc1 overexpression rescues spindle defects in Ndc80-deleted oocytes. Ndc80f/f Zp3-Cre oocytes overexpressing mEGFP-HURP, mEGFP-Kif11, mNeonGreen-HSET, or mNeonGreen-Prc1 were immunostained at metaphase I (5.5 h after NEBD). Spindle shapes were reconstructed in 3D and categorized (n = 29, 18, 18, 18, 24 oocytes from three independent experiments). ****p < 0.0001 (p = 5.1E−05) by two-tailed unpaired Student’s t-test. n.s., not significant. Scale bars, 10 μm. Mean +/− SD are presented in a–c.