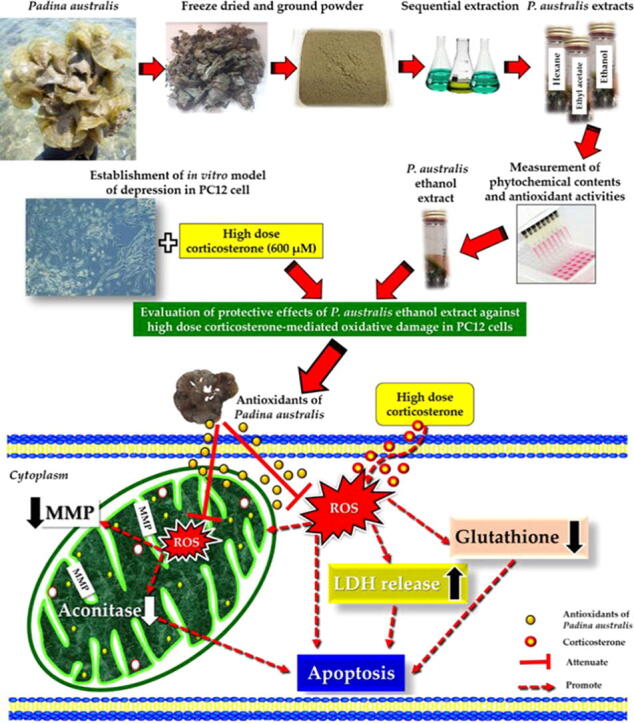
Graphical abstract
Keywords: Padina australis antioxidants, High dose corticosterone, Depression mimicking, Oxidative damage, Antidepressant-like effects
Abstract
Oxidative damage has been associated with the pathophysiology of depression. Macroalgae are equipped with antioxidant defense system to counteract the effects of free radicals. We explored the use of Malaysian Padina australis to attenuate high dose corticosterone-mediated oxidative damage in a cellular model mimicking depression. Fresh specimen of P. australis was freeze-dried and extracted sequentially with hexanes, ethyl acetate and ethanol. The extracts were screened for their phytochemical contents and antioxidant activities. Ethanol extract demonstrated the most potent antioxidant capacity and was selected for subsequent assays against high dose corticosterone of 600 µM-mediated oxidative damage in the rat pheochromocytoma (PC12) cells. The corticosterone reduced the cell viability, glutathione (GSH) level, aconitase activity, and mitochondrial membrane potential (MMP); and increased the lactate dehydrogenase (LDH) release, intracellular reactive oxygen species (ROS) level and apoptosis. However, the extent of oxidative damage was reversed by 0.25–0.5 mg/mL ethanol extract suggesting a possible role of P. australis-based antioxidants in the mitochondrial defense against constant ROS generation and regulation of antioxidant pathway. The effects were similar to that of desipramine, a tricyclic antidepressant. Our findings indicate that P. australis can be developed as a mitochondria-targeted antioxidant to mitigate antidepressant-like effects.
1. Introduction
The chronic exposure to stressful life events is an established risk factor for the development of many psychological conditions in humans, including major depression (Kendler et al., 1999). Depression is a leading cause of morbidity worldwide (Vos et al., 2012) and has a profound impact on functioning and quality of life. Depression is a complex illness with many contributing factors, including cardiovascular disease, obesity, diabetes, cancer and cognitive impairment (Black et al., 2015). Depression has been reported to affect 350 million people globally with a lifetime risk of 7% (Li et al., 2015) and it is likely to cause an increase of 5.7% in the global burden of disease by 2020 besides being predicted as the leading cause of disability by the year 2030 (Li et al., 2015, Mathers and Loncar, 2006). The prevalence of depression in Malaysia was approximately 10%. Women of low socio-economic status and individuals with comorbid mental and medical conditions showed higher vulnerabilities to experiencing depression. A survey conducted by the Ministry of Health Malaysia in 2017, revealed that 7% of the adults were at varying stages of depression and of these, 11,811 (64%), 3680 (20%) and 1682 (9%) were suffering from mild, moderate and severe depression, respectively (Lim et al., 2018). Further, there are appreciable choices of antidepressant medications available but mostly intolerable due to their unpleasant adverse effects such as headaches, nausea, insomnia, drowsiness, diarrhea, ejaculatory disorder, increased sweating, dry mouth, constipation and loss of appetite (Kennedy et al., 2016, Cipriani et al., 2018).
Pathophysiology of depression involves the monoamine neurotransmitter, hypothalamic-pituitary-adrenal (HPA) axis activation, inflammation and cytokine hypotheses. The HPA axis is mainly regulated by corticotropin-releasing hormone (CRH) secreted by the hypothalamus. The CRH stimulates the synthesis and release of adrenocorticotropic hormone (ACTH) by the pituitary gland. The ACTH, which in turn is responsible for the secretion of glucocorticoid (corticosterone in rodents) by the adrenal gland. In response to stress, corticosterone is rapidly synthesized and secreted from the adrenal cortex, resulting in the increased levels of corticosterone in plasma and the hippocampus. The HPA axis dysregulation has been reported to be one of the most prominent neurobiological changes in the etiology of depression (Mao et al., 2012). Exogenous administration of high dose corticosterone to rat pheochromocytoma PC12 cells can induce oxidative damage and cell apoptosis mimicking the symptoms of depression (Zhang et al., 2019). Normal PC12 cells possess typical neuronal-like feature and express high levels of glucocorticoid receptors (Zhou et al., 2017, Zhou et al., 2018, Gong et al., 2019).
Reactive oxygen species (ROS) is required for cellular signaling and defence against invading pathogens under normal condition. An equilibrium between ROS production and the antioxidant defenses protects cells against oxidative damage. Oxidative damage is defined as detrimental biological effects of free radicals (Pizzino et al., 2017). This equilibrium may become altered under adverse conditions including psychiatric illnesses through an excessive ROS production or impaired antioxidant defences. Excessive ROS production can cause oxidative damage to functional proteins, nucleotides, lipids, plasma membrane and cytoplasmic organelles, that eventually leads to apoptosis. Oxidative modification of deoxyribonucleic acid (DNA) induces mutation causing alterations in gene expression by influencing the availability or activity of transcription factors. The 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) and 8-oxo-7,8-dihydroguanosine (8-oxoG) generated by cellular metabolism of ROS are among the most extensively studied products of DNA oxidation (Cooke and Evans, 2007). The 8-oxodG can be assessed in nuclear or mitochondrial DNA (Guetens et al., 2002), as well as in urine (Loft and Poulsen, 1999). In a report by Behr et al. (2012), depressed patients exhibited significantly increased urinary concentrations of 8-oxodG as compared to healthy subjects suggesting the role of ROS in the pathophysiology of depression. On the other hand, 8-oxoG formed by spontaneous DNA oxidation may also cause single nucleotide polymorphisms (SNPs) in the human genome contributing to the genomic diversity in human beings. Chromosomal regions with a high density of 8-oxoG coincide with high levels of SNPs (Ohno et al., 2006). Accumulating evidence suggests that inherited SNPs in genes critical for inflammatory pathways; IL-1, IL-6, tumour necrosis factor alpha (TNF-α) and cyclooxygenase-2 (COX-2) contribute to increased risk of depression. The SNPs in the genes coding for endogenous antioxidant enzymes; catalase, gluthatione peroxidase, glutathione reductase and superoxide dismutase have also been reported to be associated with predisposition to different forms of depression (Vaváková et al., 2015). Therefore, oxidative damage caused by excessive ROS production has received substantial attention with regards to depression and has been proposed as a contributing factor to the pathophysiology of depression (Salim, 2014).
Antioxidants have been thoroughly tested for their efficacy to slow down the progressive deterioration in depression (Gautam et al., 2012, Xu et al., 2014). Antioxidants are effective against free radicals at low concentrations in the ratio of 1:100 for antioxidants and free radicals (Vaváková et al., 2015). Enzymatic antioxidants are represented by the catalase, glutathione peroxidase and superoxide dismutase. Exogenous antioxidants include the Vitamins E and C, thiol antioxidants (glutathione, thioredoxin and lipoic acid), melatonin, carotenoids and natural flavonoids. Both categories of antioxidants display synergistic and interdependent effects on one another establishing the “antioxidant network” (Kurutas, 2016). There are growing indications supporting a link between increased levels of ROS or reactive nitrogen species (RNS) and deteriorated activities of enzymatic and exogenous antioxidants in various diseases (Rahman, 2007, Valko et al., 2007, Poljsak et al., 2013, Kurutas, 2016). Upon exposure to excessive ROS, the endogenous antioxidant system is compromised and failed to offer appropriate or total protection against oxidative damage. Exogenous antioxidants in the form of food, dietary supplements or pharmaceutical substances can compensate the deficit of endogenous antioxidants. The complexity of human antioxidant defense maintains the modest level of ROS while allowing its beneficial roles to perform redox regulation of cellular signaling (Halliwell, 2011).
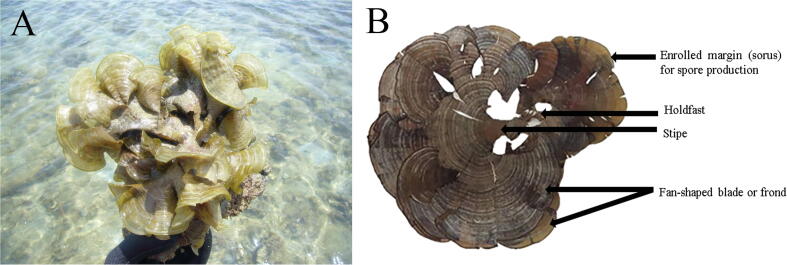
Padina australis Hauck is a species of brown macrolagae that belongs to the class of Phaeophyceae (Guiry and Guiry, 2019) (Fig. 1A). It is commonly found in tidepools on the reef flats attached to solid substrates by discoid holdfast (Fig. 1B) and thrives mainly in the tropical and subtropical waters worldwide (Trono and Ganzon-Fortes, 1988). Padina australis has been reported to possess numerous biological activities including antioxidant (Gany et al., 2014, Murugan et al., 2015), anti-neuroinflammatory (Gany et al., 2014), antimicrobial (Chong et al., 2011, Zailanie, 2016) and antiacetylcholinesterase properties (Gany et al., 2014, Murugan et al., 2015) along with stimulation of neurotrophic activity in the hippocampal neurons (Tirtawijaya et al., 2016). The therapeutic effects were attributed to antioxidant phenols (Singh and Sidana, 2013). Therefore, this study aimed to examine the protective effects of antioxidants derived from Malaysian P. australis against high dose corticosterone-mediated oxidative damage in PC12 cells mimicking symptoms of depression.
Fig. 1.
Padina australis Hauck (A) Its distribution on a coral reef at Cape Rachado, Port Dickson, Negeri Sembilan, Malaysia. (B) Morphological features of a herbarium specimen.
2. Materials and methods
2.1. Collection of P. australis and preparation of solvent extracts
Fresh specimen of P. australis was collected from Cape Rachado, Port Dickson, Negeri Sembilan, Malaysia. Species identification was based on morphological analysis of thalli that can be divided into holdfast, stipe and blade (Trono, 1997). Herbarium voucher was prepared and deposited at Sunway University, Selangor Darul Ehsan, Malaysia. Specimens were cleaned and washed with salt water to remove debris and mud, rinsed in distilled water, freeze dried (LaboGene, Brigachtal, Germany) for 48 to 72 h at −50 ± 2 °C and ground into fine powder. The powder was extracted sequentially with solvents of increasing polarity: hexanes < ethyl acetate < 95% ethanol at 1:20 ratio (w/v) and 200 rpm for 20 min at 25 °C. Supernatant was concentrated in a vacuum concentrator (LaboGene, Brigachtal, Germany). Concentrated extract was stored at −20 °C until further use. The extraction yield (%) of concentrated extract was calculated according to equation (1).
| (1) |
2.2. Evaluation of total phenolic content
Total phenolic content was measured according to the method described by Singleton et al., 1999, Pang et al., 2018, and expressed as milligram gallic acid equivalent per gram of extract (mg GAE/g). Gallic acid was used as a standard reference.
2.3. Evaluation of total flavonoid content
Total flavonoid content concentration was quantified according to the method described by Pękal and Pyrzynska, 2014, Pang et al., 2018, and expressed as milligram of quercetin equivalent per gram of extract (mg QE/g). Quercetin was used as a standard reference.
2.4. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay
Scavenging activity on DPPH radical was determined according to the method described by Brand-Williams et al. (1995), and expressed as half-maximum effective concentration (EC50) value (mg/mL) at which DPPH radicals were scavenged by 50%. Ascorbic acid was used as a positive control.
2.5. 2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) free radical scavenging assay
Scavenging activity on ABTS was determined according to the method described by Miller et al., 1993, Re et al., 1999, and expressed as half-maximal effective concentration (EC50) value (mg/mL) at which ABTS radicals were scavenged by 50%. Ascorbic acid was used as a positive control.
2.6. Reducing power assay
Reducing power was determined according to the method described by Oyaizu, 1986, Pang et al., 2018, and expressed as half-maximal effective concentration (EC50) value (mg/mL) at which the absorbance was 0.5. Ascorbic acid was used as a positive control.
2.7. PC12 cell culture
PC12 adherent cells (PC12 Adh) were a kind gift of Associate Professor Dr. William Lim Kiong Seng of University Malaysia Sarawak, Malaysia. The cells were maintained in Nutrient Mixture F-12 Ham Kaighn’s modification (F-12 K) (Sigma-Aldrich, St. Louis, MO) supplemented with 2.5% fetal bovine serum, 15% horse serum and 1% penicillin-streptomycin at 37 ± 2 °C in a 5% CO2-humidified incubator. PC12 cells in complete F-12K without treatment served as a negative control while PC12 cells treated with 10 µg/mL desipramine (Sigma-Aldrich, St. Louis, MO) served as a positive control.
2.8. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell viability assay
PC12 cells were plated at a density of 8 × 104 cells per well in a 96-well plate and incubated for 24 h at 37 ± 2 °C in a 5% CO2-humidified incubator. Supernatant was removed and replaced with fresh medium containing various concentrations of corticosterone (EMD Milipore Corporation, Temecula, CA) (Experiment 2.8.1) or various concentrations of selected P. australis extract determined based on the phytochemical contents and antioxidant activities (2.2, 2.6) and incubated for 24 h (Experiment 2.8.2). The protective effects of P. australis against high dose corticosterone-mediated cytotoxicity in PC12 cells was investigated by pre-treating the cells with selected range of concentrations determined based on Experiment 2.8.2 for 2 h followed by high dose corticosterone for another 24 h (Experiment 2.8.3). Each well was then added with 10 µL of 0.5 mg/mL MTT (Merck & Co, Rahway, NJ) and incubated for 4 h. MTT is a yellow tetrazolium salt, which is reduced to a purple formazan by dehydrogenases of a live cell. The amount of formazan produced is directly proportional to the number of live cells. Supernatant was removed and the formazan was dissolved by 100 µL dimethyl sulfoxide (DMSO). The extent of MTT reduction was determined by measuring the absorbance at 570 nm with background subtraction at 630 nm in a UV–Vis spectrophotometer microplate reader (Infinite 200 Pro, Männedorf, Switzerland) and expressed as a percentage of viable cells relative to negative control.
A high dose corticosterone determined based on Experiment 2.8.1 (600 µM) and the most optimum concentration of extract determined based on Experiment 2.8.3 (0.25 mg/mL ethanol extract) were selected for subsequent assays of oxidative damage.
2.9. Lactate dehydrogenase (LDH) assay
PC12 cells were plated at a density of 1 × 104 cells per well in a 96-well plate and incubated for 24 h at 37 ± 2 °C in a 5% CO2-humidified incubator. Supernatant was removed and pre-treated with fresh medium containing 0.25 mg/mL P. australis ethanol extract or 10 µg/mL desipramine for 2 h before exposure to 600 µM corticosterone for 24 h. Supernatant was then subjected to LDH measurement according to the manufacturer’s protocol of LDH cytotoxicity detection kit (Roche, Mannheim, Germany). The release of LDH was determined by measuring the absorbance at 492 nm with background subtraction at 690 nm in a UV–Vis spectrophotometer microplate reader and expressed as a percentage relative to negative control.
2.10. Reduced glutathione (GSH) and oxidized glutathione (GSSG) assay
PC12 cells were plated at a density of 1 × 104 cells per well in a 96-well white opaque plate and incubated for 24 h at 37 ± 2 °C in a 5% CO2-humidified incubator. Supernatant was removed and pre-treated with fresh medium containing 0.25 mg/mL P. australis ethanol extract or 10 µg/mL desipramine for 2 h before exposure to 600 µM corticosterone for 24 h. Supernatant was then subjected to GSH and GSSG measurement according to the manufacturer’s protocol of GSH/GSSG-Glo glutathione assay kit (Promega Corporation, Madison, WI) in a UV–Vis spectrophotometer microplate reader) and expressed as the ratio of GSH to GSSG.
2.11. Aconitase assay
PC12 cells were plated at a density of 1 × 106 cells per well in a 6-well plate and incubated for 24 h at 37 ± 2 °C in a 5% CO2-humidified incubator. Supernatant was removed and pre-treated with fresh medium containing 0.25 mg/mL P. australis ethanol extract or 10 µg/mL desipramine for 2 h before exposure to 600 µM corticosterone for 24 h. Cell lysate was then subjected to aconitase activity measurement according to the manufacturer’s protocol of aconitase activity kit (Sigma-Aldrich, St. Louis, MO). Aconitase activity was determined by measuring the absorbance at 450 nm in a UV–Vis spectrophotometer microplate reader and expressed as a percentage relative to negative control.
2.12. Mitochondrial membrane potential (MMP) assay
PC12 cells were plated at a density of 8 × 104 cells per well in 96-well black plate and incubated for 24 h at 37 ± 2 °C in a 5% CO2-humidified incubator. Supernatant was removed and pre-treated with fresh medium containing 0.25 mg/mL P. australis ethanol extract or 10 µg/mL desipramine for 2 h before exposure to 600 µM corticosterone for 24 h. Supernatant was then subjected to MMP measurement according to the manufacturer’s protocol of mitochondrial membrane potential kit (Sigma-Aldrich, St. Louis, MO). Fluorescence intensity shift from red to green was determined by measuring the absorbance at an excitation and emission wavelength of 540 nm/590 nm (red) and 490 nm/525 nm (green) in a UV–Vis spectrophotometer microplate reader and expressed as a percentage relative to negative control.
2.13. Intracellular reactive oxygen species (ROS) assay
The intracellular ROS level was measured according to the method described by Li et al., 2009, Mao et al., 2010 with minor modifications. PC12 cells were plated at a density of 8 × 104 cells per well in a 96-well black plate and incubated for 24 h at 37 ± 2 °C in a 5% CO2-humidified incubator. Supernatant was removed and pre-treated with fresh medium containing 0.25 mg/mL P. australis ethanol extract or 10 µg/mL desipramine for 2 h before exposure to 600 µM corticosterone for 24 h. Supernatant was then removed and replaced with 25 µM of 2′,7′–dichlorofluorescin diacetate (DCFH-DA) (Sigma-Aldrich, St. Louis, MO), incubated for 30 min at 37 ± 2 °C and washed twice with phosphate buffer saline (PBS). Green fluorescence indicated dichlorofluorescin (DCF), a product of DCFH oxidation. The intensity was measured at an excitation and emission wavelength of 485 nm/535 nm in a UV–Vis spectrophotometer microplate reader and expressed as a percentage relative to negative control.
2.14. Hoechst 33258 staining for apoptotic cells
PC12 cells were plated at a density of 8 × 104 cells per well in a 6-well plate and incubated for 24 h at 37 ± 2 °C in a 5% CO2-humidified incubator. Supernatant was removed and pre-treated with fresh medium containing 0.25 mg/mL P. australis ethanol extract or 10 µg/mL desipramine for 2 h before exposure to 600 µM corticosterone for 24 h. Supernatant was then removed and replaced with 1.0 mL of 5 μg/mL Hoechst 33258 (Invitrogen, Carlsbad, CA), incubated for 10 min at 37 ± 2 °C and washed twice with PBS. Images were acquired on Nikon Eclipse Ti-S inverted microscope and Intensilight C-HGFI Precentered Fiber Illuminator (Nikon Corporation, Tokyo, Japan) at 460 nm. Bright-blue fluorescence indicated apoptotic cells. The number of apoptotic cells was counted in five randomly selected non-overlapping fields and expressed as a percentage relative to negative control.
2.15. Statistical analysis
Statistical analysis was performed with Statistical Package for Social Sciences (SPSS, version 23.0 for Windows, Chicago, IL, USA) and the data were expressed as means ± standard deviation (SD) of three independent replicates. Shapiro-Wilk test was employed to test the normality of data distribution. Levene’s test was used for the assessment of homogeneity of variance. If the data were normally distributed, one-way ANOVA followed by Bonferroni post-hoc multiple comparison test for equal variances assumed was performed. For non-normally distributed data, Kruskal-Wallis test followed by Games-Howell post-hoc multiple comparison test for equal variances not assumed was performed. Statistical differences with p < 0.05 were considered as significant.
3. Results
3.1. Extraction yield, phytochemical contents and in vitro antioxidant activities of P. australis extracts
Table 1 shows the extraction yield (%), phytochemical contents and in vitro antioxidant activities of P. australis extracts. The yield of P. australis extracts and phytochemical contents in decreasing order are ethanol extract > ethyl acetate extract > hexane extract (p < 0.05). Total phenolic and flavonoid contents of ethanol extract were 8 to 40-fold and 15-fold higher than that of hexane and ethyl acetate extracts, respectively. Ethanol extract exhibited the highest antioxidant activities with the lowest EC50 values of 0.01 ± 0.0, 0.07 ± 0.0 and 0.21 ± 0.0 mg/mL for DPPH, ABTS and reducing power, respectively (p < 0.05). A low EC50 value indicates high antioxidant activity and vice versa. Since the ethanol extract showed most potent antioxidant activities among the tested extracts, it was selected for subsequent assays of oxidative damage.
Table 1.
Extraction yield, phytochemical contents and antioxidant activities of P. australis extracts. All data are shown as the mean ± S.D. in triplicates (n = 3). Means with different letters in the same assay are significantly different [(p < 0.05; Bonferroni (extraction yield) and Games-Howell (phytochemical contents and antioxidant activities)]. Ascorbic acid was used as a positive control.
| Padina australis extracts / Ascorbic acid | Extraction yield (%) | Total phenolic content (mg GAE/g) | Total flavonoid content (mg QE/g) | EC50 (mg/mL) |
||
|---|---|---|---|---|---|---|
| DPPH radical scavenging | ABTS radical scavenging | Reducing power | ||||
| Hexane | 1.49 ± 0.1a | 2.32 ± 1.2a | 9.74 ± 2.3a | 0.05 ± 0.0a | 0.51 ± 0.0a | 3.44 ± 0.4a |
| Ethyl acetate | 2.80 ± 0.0b | 11.64 ± 1.0b | 10.57 ± 1.2a | 0.02 ± 0.0b | 0.31 ± 0.1b | 2.94 ± 0.3a |
| Ethanol | 3.63 ± 0.0c | 92.67 ± 10.6c | 150.94 ± 12.0b | 0.01 ± 0.0c | 0.07 ± 0.0c | 0.21 ± 0.0b |
| Ascorbic acid | – | 737.91 ± 29.3d | 915.09 ± 16.6c | 0.00 ± 0.0d | 0.00 ± 0.0d | 0.01 ± 0.0c |
EC50 = half-maximum effective concentration; DPPH = 2,2-diphenyl-1-picrylhydrazyl; ABTS = 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid)
3.2. Effects of corticosterone on the viability of PC12 cells
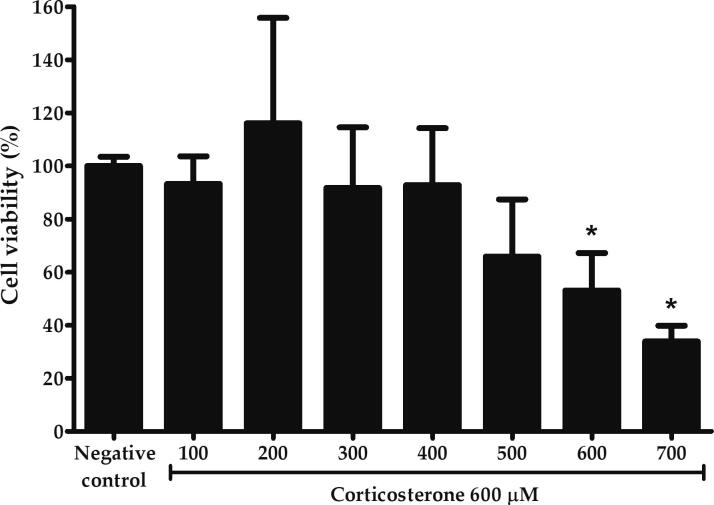
The vulnerability of PC12 cells to oxidative damage caused by dysregulation of HPA axis was investigated through exposure to corticosterone at various concentrations ranged from 100 to 700 µM. As shown in Fig. 2, the viability decreased gradually with the increasing concentrations until a sharp decline at 700 µM. At 600 and 700 µM, the viability was significantly reduced to 53.16 ± 14.08 and 34 ± 5.95%, respectively compared to negative control (p < 0.05). Since a large-scale cellular death was more pronounced by challenging the cells with 700 µM corticosterone, therefore a lower concentration of 600 µM was selected as a high dose corticosterone for the subsequent assays of oxidative damage.
Fig. 2.
Effects of corticosterone on the viability of PC12 cells evaluated by MTT assay following incubation with various concentrations of corticosterone for 24 h. Asterisk (*) symbol indicates significant difference (p < 0.05; Games-Howell) in viability relative to the negative control. All data are shown as the mean ± S.D. in triplicates (n = 3).
3.3. Effects of P. australis ethanol extract on the viability of PC12 cells
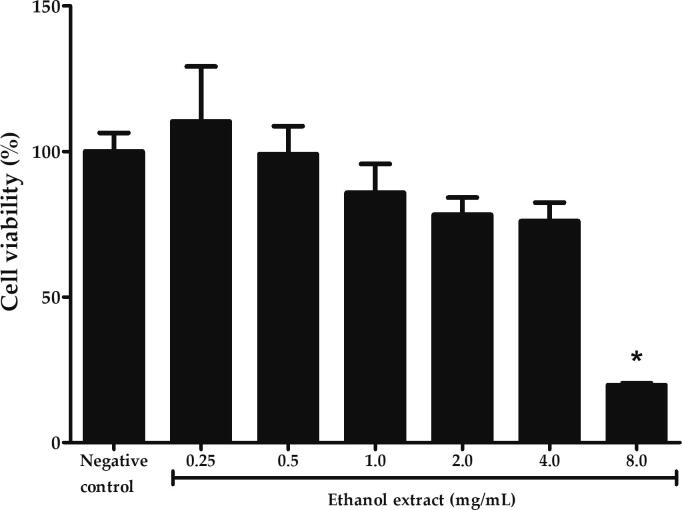
The effects of ethanol extract on the viability of PC12 cells was assessed to exclude any possibilities of cytotoxicity prior to the investigation of protective activities. As shown in Fig. 3, the viability decreased gradually with the increasing concentrations until 4 mg/mL with a sharp decline at 8 mg/mL. At 8 mg/mL, the viability was significantly reduced to 19.73 ± 0.66% compared to negative control (p < 0.05). The 50% inhibitory concentration of ethanol extract in PC12 cells was 5.40 mg/mL. There was no cytotoxicity observed in PC12 cells at the concentrations ranged from 0.25 to 4 mg/mL. Therefore, ethanol extract in the range of 0.25 to 4 mg/mL was selected for the subsequent assays of oxidative damage.
Fig. 3.
Effects of P. australis ethanol extract on the viability of PC12 cells evaluated by MTT assay following incubation with various concentrations of ethanol extract for 24 h. Asterisk (*) symbol indicates significant difference (p < 0.05; Bonferroni) in viability relative to the negative control. All data are shown as the mean ± S.D. in triplicates (n = 3).
3.4. Effects of P. australis ethanol extract on the high dose corticosterone-mediated cytotoxicity in PC12 cells
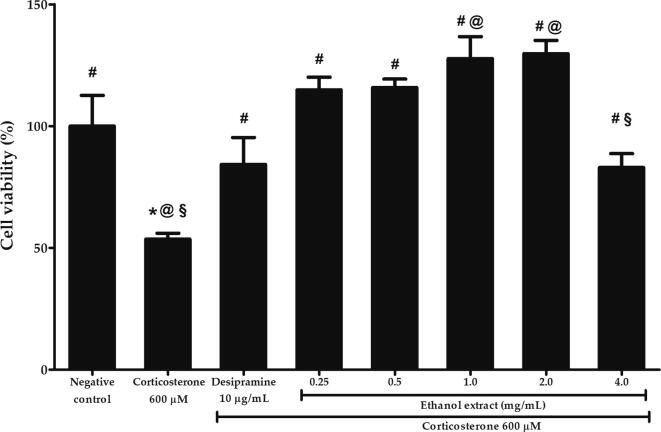
The protective effects of P. australis ethanol extract against 600 µM corticosterone-mediated cytotoxicity was investigated by pre-treating the cells with 0.25 to 4 mg/mL ethanol extract for 2 h followed by 600 µM corticosterone for another 24 h. As shown in Fig. 4, the viability was significantly reduced to 53.57 ± 2.47% by 600 µM corticosterone compared to negative control (p < 0.05). However, pre-treatment with ethanol extract in the range of 0.25 to 4 mg/mL significantly increased the viability compared to corticosterone (p < 0.05). Nevertheless, the viability was significantly lower at 4 mg/mL compared to 0.25 mg/mL ethanol extract (p < 0.05). There was no significant difference in viability between 0.25 mg/mL and 0.5 to 2.0 mg/mL ethanol extract. Therefore, the lowest concentration of 0.25 mg/mL ethanol extract was selected for the subsequent assays of oxidative damage.
Fig. 4.
Effects of P. australis ethanol extract on the viability of PC12 cells evaluated by MTT assay following pre-treatment with various concentrations of ethanol extract for 2 h and exposure to high dose corticosterone of 600 µM for 24 h. Asterisk (*), hash (#), alias (@) and section (§) symbols indicate significant difference (p < 0.05; Bonferroni) in viability relative to the negative control, corticosterone, desipramine and 0.25 mg/mL ethanol extract, respectively. All data are shown as the mean ± S.D. in triplicates (n = 3).
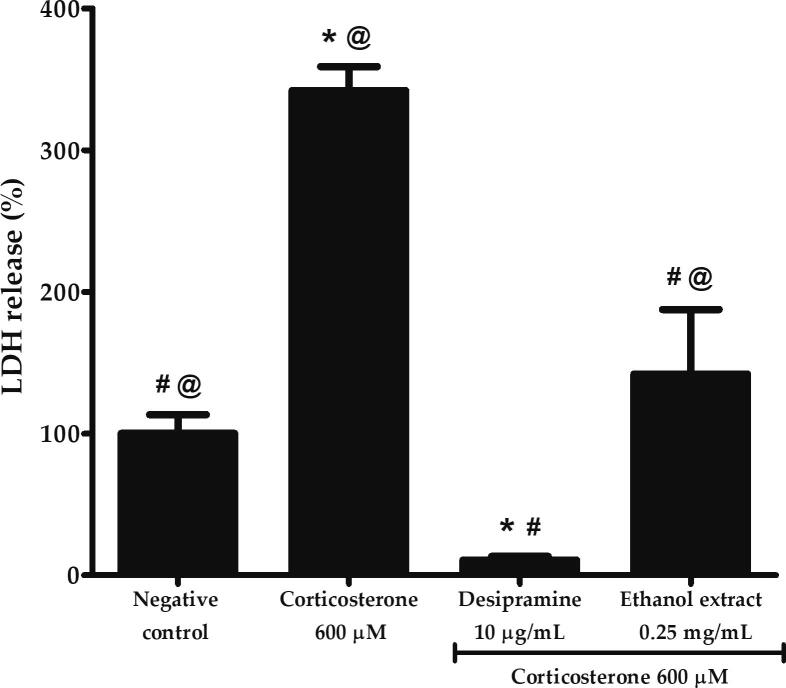
3.5. Effects of P. australis ethanol extract on the high dose corticosterone-mediated LDH release in PC12 cells
Lactate dehydrogenase is an oxidoreductase enzyme released into cytoplasm upon damage to the plasma membrane. As shown in Fig. 5, PC12 cells treated with 600 µM corticosterone released 341.88 ± 17.05% LDH, being 3.4-fold and 32-fold higher compared to negative control and desipramine, respectively (p < 0.05). However, ethanol extract significantly reduced the release of LDH to 141.91 ± 45.65 (p < 0.05) or 2.4-fold lower compared to corticosterone.
Fig. 5.
Effects of P. australis ethanol extract on LDH release following pre-treatment with 0.25 mg/mL ethanol extract for 2 h and exposure to high dose corticosterone of 600 µM for 24 h. Asterisk (*), hash (#) and alias (@) symbols indicate significant difference (p < 0.05; Games-Howell) in LDH release relative to the negative control, corticosterone and desipramine, respectively. All data are shown as the mean ± S.D. in triplicates (n = 3).
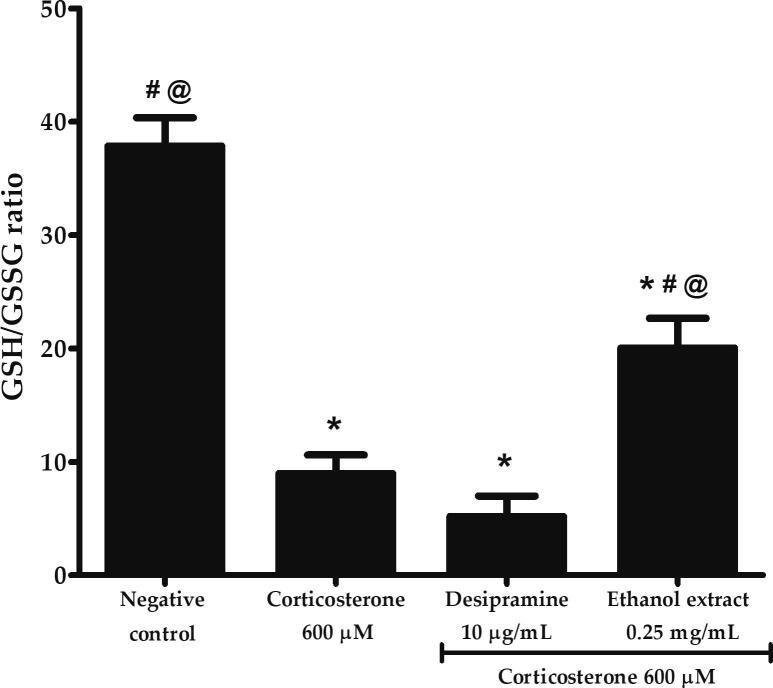
3.6. Effects of P. australis ethanol extract on high dose corticosterone-mediated glutathione depletion in PC12 cells
Glutathione is an endogenous antioxidant playing an important role in the prevention of cellular damage caused by excessive ROS production. The ratio of GSH to GSSG is an indicator of cellular health. As shown in Fig. 6, PC12 cells treated with 600 µM corticosterone significantly decreased the GSH/GSSG ratio to 8.97 ± 1.63 or 4-fold lower compared to negative control (p < 0.05). However, ethanol extract significantly increased the ratio up to 20.05 ± 2.63 or 2.2-fold and 3.9-fold higher compared to corticosterone and desipramine, respectively (p < 0.05). Our findings demonstrated that ethanol extract may exert its protective effects through intracellular glutathione synthesis.
Fig. 6.
Effects of P. australis ethanol extract on glutathione depletion following pre-treatment with 0.25 mg/mL ethanol extract for 2 h and exposure to high dose corticosterone of 600 µM for 24 h. Asterisk (*), hash (#) and alias (@) symbols indicate significant difference (p < 0.05; Bonferroni) in GSH/GSSG ratio relative to the negative control, corticosterone and desipramine, respectively. All data are shown as the mean ± S.D. in triplicates (n = 3).
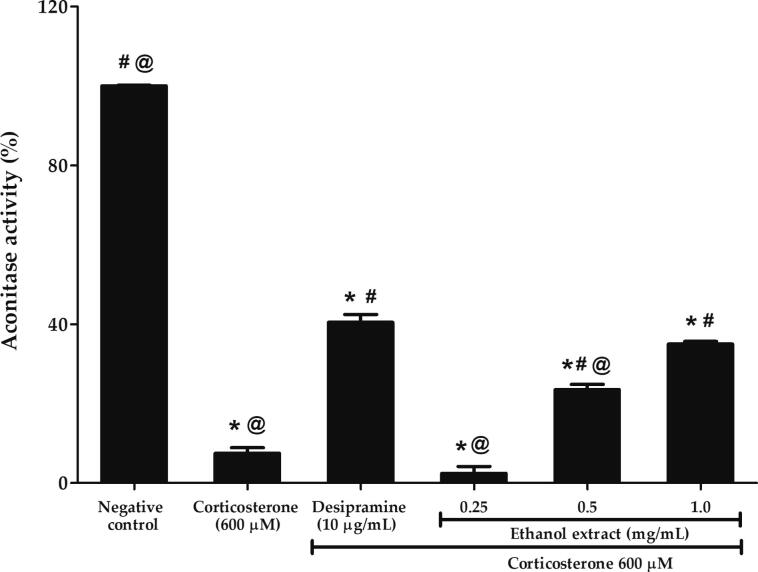
3.7. Effects of P. australis ethanol extract on the high dose corticosterone-mediated aconitase deficiency in PC12 cells
Aconitase is an iron-sulfur protein that catalyzes the reversible citrate-isocitrate conversion in Krebs cycle and is vulnerable to oxidative damage. The extreme sensitivity of aconitase to excessive ROS production is an indicator of superoxide or hydrogen peroxide (H2O2) accumulation. As shown in Fig. 7, PC12 cells treated with 600 µM corticosterone shows 92% decrease in aconitase activity at 7.47 ± 1.42% compared to negative control (p < 0.05). 0.25 mg/mL ethanol extract failed to restore the activity. Therefore, the higher concentrations of ethanol extract at 0.5 and 1.0 mg/mL were tested and aconitase activity was found to increase up to 3.1-fold at 23.49 ± 1.32% and 4.7-fold at 34.95 ± 0.72%, respectively compared to corticosterone (p < 0.05). Treatment with 1 mg/mL ethanol extract was observed to be comparable to the effect of desipramine in increasing the aconitase activity.
Fig. 7.
Effects of P. australis ethanol extract on aconitase deficiency following pre-treatment with 0.25 mg/mL ethanol extract for 2 h and exposure to high dose corticosterone of 600 µM for 24 h. Asterisk (*), hash (#) and alias (@) symbols indicate significant difference (p < 0.05; Games-Howell) in aconitase activity relative to the negative control, corticosterone and desipramine, respectively. All data are shown as the mean ± S.D. in triplicates (n = 3).
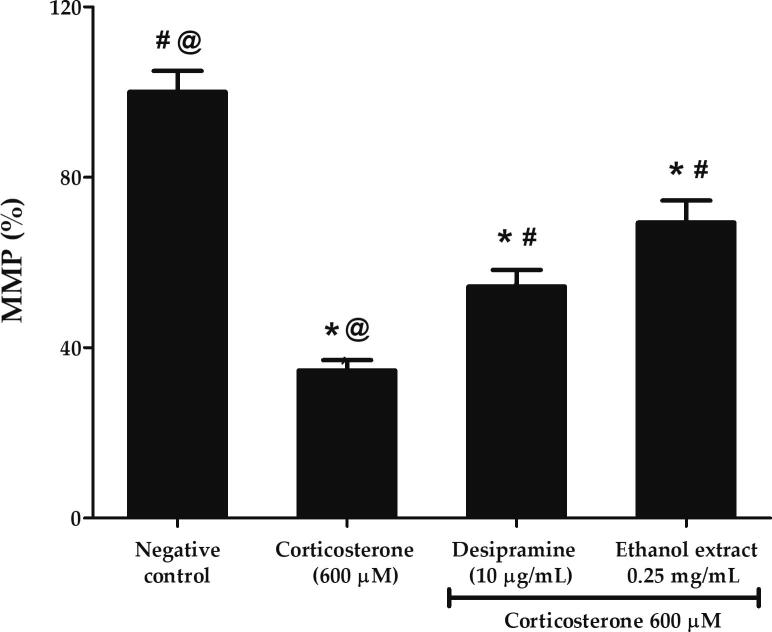
3.8. Effects of P. australis ethanol extract on the high dose corticosterone-mediated mitochondrial depolarization in PC12 cells
In response to ROS overloading, inner mitochondrial membrane is abruptly permeable to small solutes resulting in dissipation of MMP, release of apoptotic factors and eventually apoptosis. JC-10 dye facilitates the discrimination of energized and de-energized mitochondria through the formation of red fluorescence aggregates when concentrated in energized mitochondria as the membrane potential increases. As shown in Fig. 8, PC12 cells treated with 600 µM corticosterone significantly decreased the MMP to 34.69 ± 2.41% or 2.89-fold lower compared to negative control (p < 0.05). In contrast, ethanol extract increased the MMP up to 2-fold at 69.37 ± 5.19% compared to corticosterone (p < 0.05) indicating its ability to restore the mitochondrial membrane potential. Ethanol extract was observed to be comparable to the effect of desipramine in reducing the MMP depolarization.
Fig. 8.
Effects of P. australis ethanol extract on mitochondrial depolarization following pre-treatment with 0.25 mg/mL ethanol extract for 2 h and exposure to high dose corticosterone of 600 µM for 24 h. Asterisk (*), hash (#) and alias (@) symbols indicate significant difference (p < 0.05; Bonferroni) in MMP relative to the negative control, corticosterone and desipramine, respectively. All data are shown as the mean ± S.D. in triplicates (n = 3).
3.9. Effects of P. australis ethanol extract on the high dose corticosterone-mediated intracellular ROS production in PC12 cells
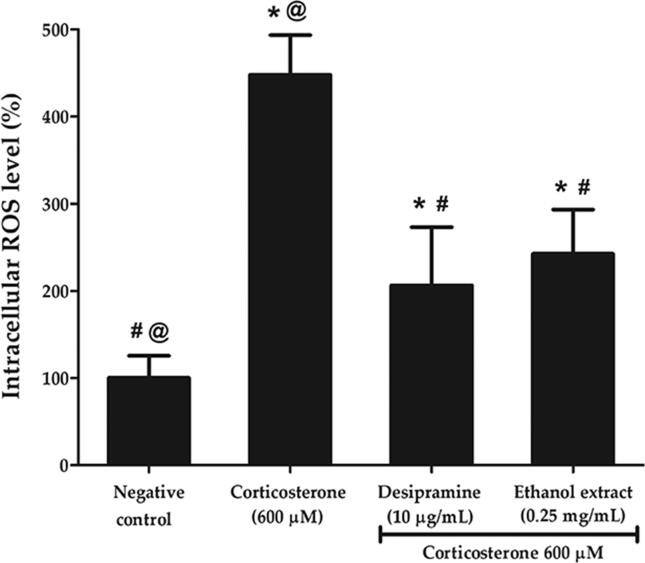
Excessive ROS production leads to mRNA damage, lipid and protein oxidation, decrease in mitochondrial function and ultimately produces more oxidative damage. Fig. 9 depicts that corticosterone significantly increased the intracellular ROS level from 100.00 ± 7.51% to 448.03 ± 45.31% (p < 0.05). The intensity was reduced to 242.59 ± 50.75% or 1.8-fold lower in ethanol extract-treated and 206.35 ± 66.88% or 2.2-fold lower in desipramine-treated groups compared to corticosterone-treated group (p < 0.05). Ethanol extract was observed to be comparable to the effect of desipramine at attenuating the intracellular ROS level.
Fig. 9.
Effects of P. australis ethanol extract on intracellular ROS production following pre-treatment with 0.25 mg/mL ethanol extract for 2 h and exposure to high dose corticosterone of 600 µM for 24 h. The graph showing intensity of DCF green fluorescence (%) in PC12 cells. Asterisk (*), hash (#) and alias (@) symbols indicate significant difference (p < 0.05; Games-Howell) in fluorescence intensity relative to the negative control, corticosterone and desipramine, respectively. All data are shown as the mean ± S.D. in triplicates (n = 3).
3.10. Effects of P. australis ethanol extract on the high dose corticosterone-mediated nuclear apoptosis in PC12 cells
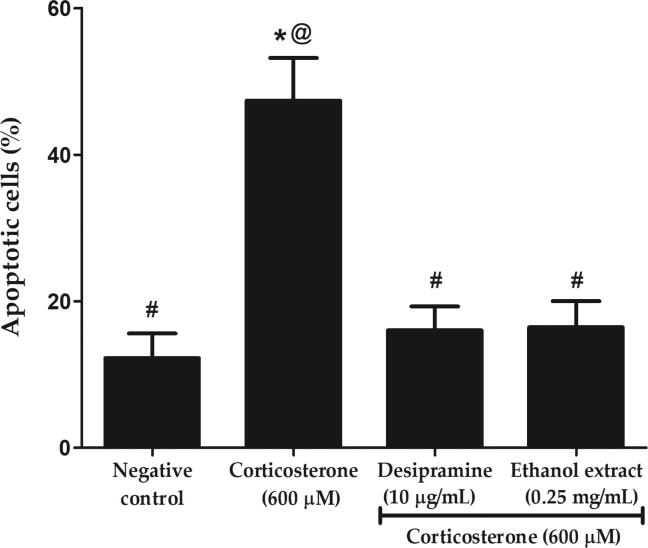
Hoechst stain is a cell-permeable dye and fluoresce strongly upon binding to DNA in live or fixed cells, thus distinguishes apoptotic cells from the normal cells based on the nuclear chromatin condensation and fragmentation. Fig. 10 depicts that corticosterone significantly increased the percentage of apoptotic cells from 12.25 ± 3.34% to 47.34 ± 5.84% (p < 0.05). Ethanol extract and desipramine reduced the percentage of apoptotic cells to 16.04 ± 3.25 and 16.46 ± 3.57%, respectively or 2.9-fold lower compared to corticosterone (p < 0.05). Similar to the findings of MMP and intracellular ROS level, ethanol extract was observed to be comparable to the effect of desipramine in reducing cellular apoptosis.
Fig. 10.
Effects of P. australis ethanol extract on nuclear apoptosis following pre-treatment with 0.25 mg/mL ethanol extract for 2 h and exposure to high dose corticosterone of 600 µM for 24 h. The graph showing percentage of apoptotic PC12 cells. Asterisk (*), hash (#) and alias (@) symbols indicate significant difference (p < 0.05; Bonferroni) in the percentage of apoptotic cells compared to negative control, corticosterone and desipramine, respectively. All data are shown as the mean ± S.D. in triplicates (n = 3).
4. Discussion
The use of natural remedies or nutraceuticals are of great importance and has been widely accepted rapidly across the world (Ekor, 2014). There are currently no studies investigated the effect of macroalgae in the in vitro model of depression linking oxidative damage to mitochondrial function. This circumstance stimulated our interest in exploring P. australis to probe the mechanism of action underlying the protective effects in depression.
Accumulating studies have revealed that the phenolic content is associated with antioxidant activities due to their redox properties and therefore contributing to the dynamic roles of reducing agents, hydrogen donors and singlet oxygen quenchers (Tenorio-Rodriguez et al., 2017, Fernando et al., 2016, Karuppusamy and Thangaraj, 2014). Marine macroalgae have attracted much attention due to their abundant secondary metabolites, mainly the phlorotannins, a highly hydrophilic polymers of phloroglucinol unique to brown macroalage (Targett and Arnold, 1998) which has been implicated in cellular defense against oxidative damage (Li et al., 2017). The total phenolic content of P. australis ethanol extract was significantly higher than the low-polarity ethyl acetate and hexane extracts, and 18.3-fold greater than that of Gracilaria manilaensis, a red macroalga (Pang et al., 2018). In contrast to a finding by Murugan et al. (2015), the total phenolic content of low-polarity n-butanol fraction of P. australis grown in Mersing, Johor, Malaysia was 188 mg GAE/g or 2-fold higher than the high-polarity ethanol extract of P. australis obtained in this study. The discrepancy may be due to genetic variation and phenolic composition of P. australis of different geographical locations. Additionally, extraction procedure, particle size, storage conditions and time, and presence of interfering substances in the extracts may contribute to variation in test results (Mekinić et al., 2019). Our observation is in agreement with the results of Wang et al. (2009) who had reported that brown macroalgae contain higher polyphenols than the red and green macroalgae grown in Icelandic waters.
Flavonoid is a class of polyphenolic compound having a benzo-γ-pyrone structure and is widely distributed in marine plants. The secondary metabolite has been thoroughly investigated due to its ability to scavenge free radicals and strongly chelate prooxidant metal ions based on functional hydroxyl groups (Leopoldini et al., 2006). Similarly, the total flavonoid content of P. australis ethanol extract was also higher than that of G. manilaensis (Pang et al., 2018). Moreover, P. australis ethanol extract also exhibited approximately 20- and 56-fold higher antioxidant activities than dichloromethane and methanol extracts as reported by Gany et al. (2014) with IC50 values of 0.276 mg/mL and 0.649 mg/mL in the DPPH radical scavenging activity, respectively, and EC50 values of 1.4 mg/mL and 1.392 mg/mL in the ABTS radical scavenging activity, respectively. Additionally, P. australis ethanol extract also demonstrated higher antioxidant activities compared to that of G. manilaensis (Pang et al., 2018).
Our findings are in accordance with Murugan et al., 2015, Pang et al., 2018 in which they observed solvents with high polarity exhibited more potent antioxidant activities compared to low polarity solvents, revealing that antioxidant compounds are often concentrated in more polar solvents (Jiang et al., 2015). Polar solvents are frequently used for recovering polyphenols from natural products. Ethanol has been regarded as the most suitable extracting solvent due to its higher polarity index bodies to ensure total extractable phenolic, flavonoid and antioxidant contents (Do et al., 2014). The gathered data demonstrated that P. australis ethanol extract contains a substantial amount of phytochemicals, indicating that pre-treatment of PC12 cells with the extract prior to corticosterone exposure exerted its protective effects through free radical scavenging by increasing the cell survival, glutathione level, aconitase activity and MMP as well as reducing the levels of LDH release and intracellular ROS.
Growing evidences have revealed that increased level of circulating corticosterone in the brain has been linked to neurotoxicity and depressive-like behaviors in mice models (Zhang et al., 2015, Bai et al., 2018). Corticosterone at 100 to 500 µM did not cause prominent cell death as the viability was above 60%. In order to observe specific changes in the cells, it was necessary to increase the treatment concentrations of corticosterone which are within an acceptable range. High dose corticosterone has been employed to induce oxidative damage in primary cultured hippocampal neurons (Zheng et al., 2018) and PC12 cells (Zhang et al., 2019). Consistently, corticosterone at 200 µM (Mao et al., 2012), 250 µM (Liu et al., 2014) and 400 µM (Wu et al., 2018) have been reported to induce oxidative damage in PC12 cells based on 50% viability after 48 to 72 h of incubation. In contrast to our study, a higher concentration of 600 µM was employed to achieve 50% viability indicating that lower concentration may require prolonged incubation period to exert cytotoxicity in PC12 cells after 24 h of incubation. It was corresponded to the damage of plasma membrane, depletion of glutathione, deficiency in aconitase, loss of MMP and increased intracellular ROS level. Basic understanding of the impact of high dose corticosterone on dynamic cellular interaction in animal models could be impeded due to high complexity of the brain. Our in vitro findings assist in understanding the onset of some key events related to the cytotoxicity of corticosterone.
Cellular mechanisms of corticosterone-mediated oxidative damage in PC12 cells involves the generation of ROS (Liu et al., 2014, Zhou et al., 2018), introduction of mitochondrial dysfunction (Li et al., 2014) and fragmentation of DNA leading to apoptosis (Liu et al., 2014, Zhou et al., 2018, Gong et al., 2019). Exposure to a high level of ROS causes deleterious consequences to the mitochondrial DNA. This work sought to tap the beneficial effect of P. australis in the mitigation of oxidative damage. Protective effects of P. australis ethanol extract might be mediated by non-enzymatic antioxidant defense system comprises glutathione, phenolics and flavonoids. Glutathione is a protein thiol molecule playing a crucial role in preventing cellular damage caused by ROS. Reduced level of glutathione causes irreversible inhibition of γ-glutamylcysteine synthetase, the rate-limiting enzyme in the biosynthesis of glutathione after exposure to high dose corticosterone. A chronic deficit in the GSH/GSSG ratio is an indicator of increased vulnerability to oxidative damage during aging, multiple chronic diseases and cataracts (Yazdanparast et al., 2008). Our results demonstrated that the ratio was significantly increased after pre-treatment with P. australis ethanol extract compared to that of desipramine. Additionally, Rosa et al. (2014) found that bipolar patients had significantly lower levels of total glutathione and it was oxidized to a greater extent. Plasma glutathione appears to be a suitable biomarker for detecting underlying oxidative damage and for evaluating the efficacy of antioxidant intervention studies.
On the other hand, mitochondria play a crucial role in maintaining cell homeostasis as well as being a major source of energy production. Mitochondrial damage is an early event during apoptosis (Petronilli et al., 2001) and is concomitant with changes in aconitase activity, MMP and production of intracellular ROS in apoptotic cells (Kim et al., 2004). The effect of glutathione on the superoxide-sensitive iron-sulfur cluster protein-containing aconitase has been explored. The primary role of mitochondrial aconitase is to control cellular ATP production through the regulation of intermediate flux in the Krebs cycle. Aconitase activity can be affected by the depletion of glutathione with concomitant generation of ROS (Song et al., 2006). Aconitase has previously been reported to be sensitive to oxidants as compared to succinate dehydrogenase (Keyer and Imlay, 1997). In this study, aconitase activity was decreased profoundly by 600 µM corticosterone and therefore confirmed the stipulated fact that the enzyme is extremely sensitive to ROS (Lodi et al., 2006). Similarly, Richardson et al., 2012, LEW et al., 2019 had observed a significant reduction of glutathione and aconitase activities in L-buthionine sulfoximine-induced oxidative damage in human Friedreich’s ataxia fibroblasts through γ-glutamylcysteine synthetase inhibition. Our findings show that 0.25 mg/mL ethanol extract failed to increase the aconitase activity although the same concentration was able to attenuate the generation of ROS. However, increased concentrations of ethanol extract at 0.5 and 1.0 mg/mL were able to restore the activity.
Moreover, MMP reflecting the mitochondrial function was observed to be dissipated by high dose corticosterone in the current study and also well supported by Li et al., 2014, Wu et al., 2018, Gong et al., 2019. Excessive ROS production induced a rapid depolarization of mitochondrial inner membrane potential and subsequent impairment of oxidative phosphorylation. Damaged mitochondria produce more ROS, in the form of superoxide anion (O2−) and H2O2. Conversely, pre-treatment with P. australis ethanol extract attenuated the dissipation of MMP. Our results suggest that P. australis ethanol extract was able to restore the MMP, a key indicator of mitochondrial activity. MMP denotes the process of electron transport and oxidative phosphorylation, and the main driving force for ATP production (Zhao et al., 2019).
Oxidative damage caused by HPA axis dysregulation involves overproduction of ROS or inadequate activity of the cellular antioxidant defenses. Excessive ROS production and mitochondrial dysfunction trigger the early events of apoptosis. Morphological changes associated with apoptosis are cell shrinkage, chromatin condensation and formation of apoptotic bodies leading to the changes in plasma membrane permeability and plasma membrane damage (Elmore, 2007). The MTT and LDH assays are employed to evaluate cell viability and apoptosis based on the activity of specific enzymes. Mitochondrial succinate dehydrogenase catalyzes the enzymatic reduction of MTT to MTT-formazan in living cells. Therefore, MTT assay is dependent on mitochondrial respiration and indicative of cellular energy capacity. In contrast, LDH is a soluble cytoplasmic enzyme and is rapidly released into the supernatant after destruction of the plasma membrane, a key feature of cells undergoing apoptosis (Kumar et al., 2018).
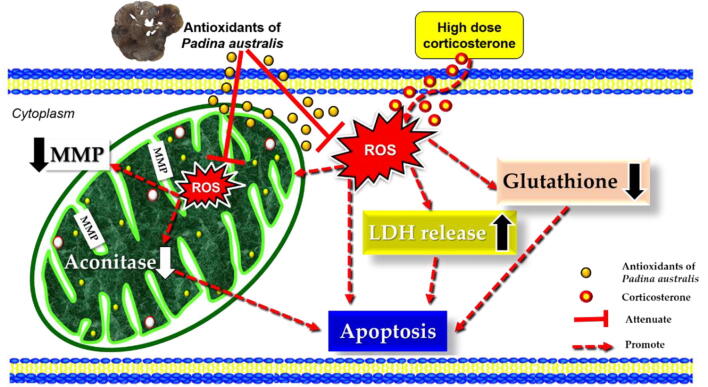
The protective mechanism of P. australis ethanol extract is presented in Fig. 11. We postulated that antioxidant substances of P. australis ethanol extract possessed protective effects against oxidative damage in PC12 cells. The antioxidant substances move across the plasma membrane into the cytoplasm and mitochondria. Upon exposure to high dose corticosterone, antioxidant substances decreased the intracellular ROS level and LDH release, prevented MMP depolarization in the inner membrane, increased the cytoplasmic glutathione level and promoted aconitase activity in the mitochondrial matrix. Restoration of the mitochondrial function prevented the activation of downstream apoptotic cascade in the maintenance of cellular integrity. Interestingly, the protective effects were similar to that of desipramine, a secondary amine tricyclic antidepressant and a potent inhibitor of the noradrenaline reuptake.
Fig. 11.
Proposed protective effects of antioxidants derived from Malaysian P. australis against high dose corticosterone-mediated oxidative damage in PC12 cells mimicking symptoms of depression.
5. Conclusions
Taken together, our current study revealed the protective effects of antioxidants derived from Malaysian P. australis against high dose corticosterone-mediated oxidative damage in a cellular model mimicking depression. The antioxidants can contribute to the attenuation of deleterious effects of ROS or acting as ROS scavengers. Hence, P. australis can be developed as a mitochondria-targeted antioxidant in the treatment of depression. As with other therapeutics for depression, the regulatory mechanisms of oxidative damage in clinical trials are highly warranted. It is vital to ensure that alternative and complementary therapeutic conforms to the required standards of safety, quality and efficacy according to the drug regulatory framework.
Acknowledgments
Acknowledgments
The authors thank Mr. Pang Jun Rui and Ms. Ngu Ee Ling of Sunway University and Ms. Lew Sze Yuen of University of Malaya for guidance in operating laboratory equipments and statistical analysis.
Funding
This work was supported by University of Malaya Research Grant, Malaysia (RP017D-14AFR) and Sunway University Internal Grant, Malaysia (INT-2019-SST-DBS-04).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Footnotes
Peer review under responsibility of King Saud University.
References
- Bai Y., Song L., Dai G., Xu M., Zhu L., Zhang W., Ju W. Antidepressant effects of magnolol in a mouse model of depression induced by chronic corticosterone injection. Steroid. 2018;135:73–78. doi: 10.1016/j.steroids.2018.03.005. [DOI] [PubMed] [Google Scholar]
- Behr G.A., Moreira J.C.F., Frey B.N. Preclinical and clinical evidence of antioxidant effects of antidepressant agents: implications for the pathophysiology of major depressive disorder. Oxid. Med. Cell. Longev. 2012;2012 doi: 10.1155/2012/609421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black C.N., Bot M., Scheffer P.G., Cuijpers P., Penninx B.W. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology. 2015;51:164–175. doi: 10.1016/j.psyneuen.2014.09.025. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995;28(1):25–30. [Google Scholar]
- Chong C.W., Hii S.L., Wong C.L. Antibacterial activity of Sargassum polycystum C. Agardh and Padina australis Hauck (Phaeophyceae) Afr. J. Biotechnol. 2011;10(64):14125–14131. [Google Scholar]
- Cipriani A., Furukawa T.A., Salanti G., Chaimani A., Atkinson L.Z., Ogawa Y., Leucht S., Ruhe H.G., Turner E.H., Higgins J.P.T., Egger M., Takeshima N., Hayasaka Y., Imai H., Shinohara K., Tajika A., Ioannidis J.P.A., Geddes J.R. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391:1357–1366. doi: 10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke, M.S., Evans, M.D., 2007. 8-oxo-deoxyguanosine: reduce, reuse, recycle? PNAS 104 (34), 13535–13536. [DOI] [PMC free article] [PubMed]
- Do Q.D., Angkawijaya A.E., Tran-Nguyen P.L., Huynh L.H., Soetaredjo F.E., Ismadji S., Ju Y. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014;22(3):296–302. doi: 10.1016/j.jfda.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014;4(177):1–9. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando S.I.P., Kim M., Kwang-Tae S., Jeong Y., You-Jin J. Antioxidant activity of marine algal polyphenolic compounds: a mechanistic approach. J. Med. Food. 2016;19(7):1–14. doi: 10.1089/jmf.2016.3706. [DOI] [PubMed] [Google Scholar]
- Gany S.A., Tan S.C., Gan S.Y. Antioxidative, anticholinesterase and anti-neuroinflammatory properties of Malaysian brown and green seaweeds. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2014;8(11):1269–1275. [Google Scholar]
- Gautam M., Agrawal M., Gautam M., Sharma P., Gautam A.S., Gautam S. Role of antioxidants in generalised anxiety disorder and depression. Indian J. Psychiatry. 2012;54(3):244–247. doi: 10.4103/0019-5545.102424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong W.X., Zhou Y.Z., Qin X.M., Du G.H. Involvement of mitochondrial apoptotic pathway and MAPKs/NF-ĸB inflammatory pathway in the neuroprotective effect of atractylenolide III in corticosterone-induced PC12 cells. Chin. J. Nat. Med. 2019;17(4):264–274. doi: 10.1016/S1875-5364(19)30030-5. [DOI] [PubMed] [Google Scholar]
- Guetens G., De Boeck G., Higley M., van Oosterom A.T., de Bruijn E.A. Oxidative DNA damage: biological significance and methods of analysis. Crit. Rev. Clin. Lab. Sci. 2002;39(4–5):331–457. doi: 10.1080/10408360290795547. [DOI] [PubMed] [Google Scholar]
- Guiry, M.D., Guiry, G.M., 2019. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway (taxonomic information republished from Algae Base with permission of M.D. Guiry). Padina australis Hauck, 1887. http://www.marinespecies.org/aphia.php?p=taxdetails&id=372010 (accessed on 12 October 2019).
- Halliwell B. Free radicals and antioxidants-quo vadis. Trends Pharmacol. Sci. 2011;32(3):125–130. doi: 10.1016/j.tips.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Li Z., Liu Y., Liu X., Chang Q., Liao Y., Pan R. Neuroprotective effect of water extract of Panax ginseng on corticosterone-induced apoptosis in PC12 cells and its underlying molecule mechanisms. J. Ethnopharmacol. 2015;159:102–112. doi: 10.1016/j.jep.2014.10.062. [DOI] [PubMed] [Google Scholar]
- Karuppusamy A., Thangaraj P. Evaluation of phenolic content, antioxidant activity, and nutritional composition of Cordia evolution (Clarke) Gamble. Int. J. Food Prop. 2014;17(1):226–238. doi: 10.1080/10942912.2011.619294. [DOI] [Google Scholar]
- Kennedy S.H., Lam R.W., McIntyre R.S., Touriman V., Bhat V., Blier P., Hasnain M., Jollant F., Levitt A.J., MacQueen G.M., Mclnerney S.J. Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder. Can. J. Psychiatry. 2016;61(9):540–560. doi: 10.1177/0706743716659417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K.S., Karkowski L.M., Prescott C.A. Causal relationship between stressful life events and the onset of major depression. Am. J. Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Keyer K., Imlay J.A. Inactivation of dehydratase [4Fe-4S] clusters and disruption of iron homeostasis upon cell exposure to peroxynitrite. J. Biol. Chem. 1997;272:27652–27659. doi: 10.1074/jbc.272.44.27652. [DOI] [PubMed] [Google Scholar]
- Kim W.H., Park W.B., Gao B., Jung M.H. Critical role of reactive oxygen species and mitochondrial membrane potential in Korean mistletoe lectin-induced apoptosis in human hepatocarcinoma cells. Mol. Pharmacol. 2004;66:1383–1396. doi: 10.1124/mol.104.001347. [DOI] [PubMed] [Google Scholar]
- Kumar P., Nagarajan A., Uchil P.D. Analysis of cell viability by the lactate dehydrogenase assay. Cold Spring Harb. Protoc. 2018;6 doi: 10.1101/pdb.prot095497. [DOI] [PubMed] [Google Scholar]
- Kurutas E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr. J. 2016;15(1):22. doi: 10.1186/s12937-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopoldini M., Russo N., Chiodo S., Toscano M. Iron chelation by the powerful antioxidant flavonoid quercetin. J. Agric. Food Chem. 2006;54(17):6343–6351. doi: 10.1021/jf060986h. [DOI] [PubMed] [Google Scholar]
- Lew S.Y., Yow Y.Y., Lim L.W., Wong K.H. Antioxidant-mediated protective role of Hericium erinaceus (Bull.: Fr.) Pers. against oxidative damage in fibroblasts from Friedreich’s ataxia patient. Food Sci. Technol. 2019 doi: 10.1590/fst.09919. [DOI] [Google Scholar]
- Li Y., Fu X., Duan D., Liu X., Xu J., Gao X. Extraction and identification of phlorotannins from the brown alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs. 2017;15(2):49. doi: 10.3390/md15020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Qian Z.J., Ryu B., Lee S.H., Kim M.M., Kim S.K. Chemical components and its antioxidant properties in vitro: an edible marine brown alga, Ecklonia cava. Bioorg. Med. Chem. 2009;17(5):1963–1973. doi: 10.1016/j.bmc.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Li Z., Li Y., Chen L., Chen P., Hu Y. Prevalence of depression in patients with hypertension: a systematic review and meta-analysis. Medicine. 2015;94(31):e1317. doi: 10.1097/md.0000000000001317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.Y., Jiang Y.M., Liu Y.M., Guo Z., Shen S.N., Liu X.M., Pan R.L. Saikosaponin D acts against corticosterone-induced apoptosis via regulation of mitochondrial GR translocation and a GR-dependent pathway. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2014;53:80–89. doi: 10.1016/j.pnpbp.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Lim G.Y., Tam W.W., Lu Y., Ho C.S., Zhang M.W., Ho R.C. Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci. Rep. 2018;8(1):2861. doi: 10.1038/s41598-018-21243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Shen S., Li Z., Jiang Y., Si J., Chang Q., Liu X., Pan R. Cajaninstilbene acid protects corticosterone-induced injury in PC12 cells by inhibiting oxidative and endoplasmic reticulum stress-mediated apoptosis. Neurochem. Int. 2014;78:43–52. doi: 10.1016/j.neuint.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Lodi A., Tonon C., Calabrese V., Schapira A.H.V. Friedreich’s ataxia: from disease mechanism to therapeutic interventions. Antioxid. Redox Signal. 2006;8:438–443. doi: 10.1089/ars.2006.8.438. [DOI] [PubMed] [Google Scholar]
- Loft S., Poulsen H.E. Markers of oxidative damage to DNA: antioxidants and molecular damage. Methods Enzymol. 1999;300:166–184. doi: 10.1016/s0076-6879(99)00124-x. [DOI] [PubMed] [Google Scholar]
- Mao Q.Q., Huang Z., Ip S.P., Xian Y.F., Che C.T. Protective effects of piperine against corticosterone-induced neurotoxicity in PC12 cells. Cell. Mol. Neurobiol. 2012;32:531–537. doi: 10.1007/s10571-011-9786-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Q.Q., Xian Y.F., Ip S.P., Tsai S.H., Che C.T. Protective effects of peony glycosides against corticosterone-induced cell death in PC12 cells through antioxidant action. J. Ethnopharmacol. 2010;133(3):1121–1125. doi: 10.1016/j.jep.2010.11.043. [DOI] [PubMed] [Google Scholar]
- Mathers C.D., Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekinić I.G., Skroza D., Šimat V., Hamed I., Čagalj M., Popović Perković Z. Phenolic content of brown algae (Pheophyceae) species: extraction, identification, and quantification. Biomolecules. 2019;9(6):244. doi: 10.3390/biom9060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N.J., Rice-Evans C., Davies M.J., Gopinathan V., Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993;84(4):407–412. doi: 10.1042/cs0840407. [DOI] [PubMed] [Google Scholar]
- Murugan, A.C., Vallal, D., Karim, M.R., Govidan, N., Yusoff, M.B.M., Rahman, M.M., 2015. In vitro antiradical and neuroprotective activity of polyphenolic extract from marine algae Padina australis H. J. Chem. Pharm. Res. 7 (8), 355–362.
- Ohno M., Miura T., Furuichi M., Tominaga Y., Tsuchimoto D., Sakumi K., Nakabeppu Y. A genomewide distribution of 8-oxoguanine correlates with the preferred regions for recombination and single nucleotide polymorphism in the human genome. Genome Res. 2006;16(5):567–575. doi: 10.1101/gr.4769606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyaizu M. Studies on products of browning reaction: antioxidative activities of product of browning reaction prepared from glucosamine. Japan J. Nutr. 1986;44(6):307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Pang J.R., Goh V.M.J., Tan C.Y., Phang S.M., Wong K.H., Yow Y.Y. Neuritogenic and in vitro antioxidant activities of Malaysian Gracilaria manilaensis Yamamoto & Trono. J. Appl. Phycol. 2018;30(6):3253–3260. doi: 10.1007/s10811-018-1438-x. [DOI] [Google Scholar]
- Pękal A., Pyrzynska K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Methods. 2014;7(9):1776–1782. [Google Scholar]
- Petronilli V., Penzo D., Scorrano L., Bernardi P., Di Lisa F. The mitochondrial permeability transition, release of cytochrome c and cell death. Correlation with the duration of pore openings in situ. J. Biol. Chem. 2001;276(15):12030–12034. doi: 10.1074/jbc.M010604200. [DOI] [PubMed] [Google Scholar]
- Pizzino G., Irrera N., Cucinotta M., Pallio G., Mannino F., Arcoraci V., Squadrito F., Altavilla D., Bitto A. Oxidative stress: harms and benefits for human health. Oxid. Medicine Cel. Longev. 2017;8416763 doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poljsak B., Šuput D., Milisay I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell. Longev. 2013;2013 doi: 10.1155/2013/956792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman K. Studies on free radicals, antioxidants and co-factors. Clin. Interv. Aging. 2007;2:219–236. [PMC free article] [PubMed] [Google Scholar]
- Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26(9–10):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Richardson T.E., Amanda E.Y., Wen Y., Yang S.H., Simpkins J.W. Estrogen prevents oxidative damage to the mitochondria in Friedreich’s Ataxia skin fibroblasts. PLoS ONE. 2012;7(4):e34600. doi: 10.1371/journal.pone.0034600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa A.R., Singh N., Whitaker E., de Brito M., Lewis A.M., Vieta E., Churchill G.C., Geddes J.R., Goodwim G.M. Altered plasma glutathione levels in bipolar disorder indicates higher oxidative stress; a possible risk factor for illness onset despite normal brain-derived neurotrophic factor (BDNF) levels. Psychol. Med. 2014;44(11):2409–2418. doi: 10.1017/S0033291714000014. [DOI] [PubMed] [Google Scholar]
- Salim S. Oxidative stress and psychological disorders. Curr. Neuropharmacol. 2014;12(2):140–147. doi: 10.2174/1570159X11666131120230309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh I.P., Sidana J. Phlorotannins. In: Dominguez H., editor. Functional Ingredients from Algae for Foods and Nutraceuticals. Woodhead Publishing; Philadelphia: 2013. pp. 187–196. [Google Scholar]
- Singleton V.L., Orthofer R., Lamuela-Raventos R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- Song J.Y., Cha J., Lee J., Roe J.H. Glutathione reductase and a mitochondrial thioredoxin play overlapping roles in maintaining iron-sulfur enzymes in fission yeast. Eukaryot. Cell. 2006;5(11):1857–1865. doi: 10.1128/EC.00244-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targett N.M., Arnold T.M. Minireview-Predicting the effects of brown algal phlorotannins on marine herbivores in tropical and temperate oceans. J. Phycol. 1998;34(2):195–205. doi: 10.1046/j.1529-8817.1998.340195.x. [DOI] [Google Scholar]
- Tenorio-Rodriguez P.A., Murillo-Álvarez J.I., Campa-Cordova Á.I., Angulo C. Antioxidant screening and phenolic content of ethanol extracts of selected Baja California Peninsula macroalgae. J. Food Sci. Technol. 2017;54(2):422–429. doi: 10.1007/s13197-016-2478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirtawijaya G., Mohibbullah M., Meinita M.D.N., Moon I.S., Hong Y.K. The ethanol extract of the rhodophyte Kappaphycus alvarezii promotes neurite outgrowth in hippocampal neurons. J. Appl. Phycol. 2016;28(4):2515–2522. [Google Scholar]
- Trono G.C., Jr., Ganzon-Fortes E.T. National Book Store; Manilla: 1988. Philippine Seaweeds. [Google Scholar]
- Trono G.C., Jr. Bookmark, Inc.; Philippines: 1997. Field Guide and Atlas of the Seaweed Resources of the Philippines. [Google Scholar]
- Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Vaváková M., Ďuračková Z., Trebatická J. Markers of oxidative stress and neuroprogression in depression disorder. Oxid. Med. Cell. Longev. 2015;2015:898393. doi: 10.1155/2015/898393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos T., Flaxman A.D., Magahi M., Lozano R., Michaud C., Ezzati M., Shibuya K., Salomon J.A., Abdalla S., Aboyans V., Abraham J. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Jónsdóttir R., Ólafsdóttir G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009;116(1):240–248. doi: 10.1016/j.foodchem.2009.02.041. [DOI] [Google Scholar]
- Wu J., Huang F.X., Wang J., Shi C.C., Fang G.Y. Protective effect of liquiritin on corticosterone-induced neurotoxicity in PC12 cells. Trop. J. Pharm. Res. 2018;17(10):2013–2017. doi: 10.4314/tjpr.v17i10.17. [DOI] [Google Scholar]
- Xu Y., Wang C., Klabnik J.J., O'Donnell J.M. Novel therapeutic targets in depression and anxiety: antioxidants as a candidate treatment. Curr. Neuropharmacol. 2014;12(2):108–119. doi: 10.2174/1570159X11666131120231448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdanparast R., Bahramikia S., Ardestani A. Nasturtium officinale reduces oxidative stress and enhances antioxidant capacity in hypercholesterolaemic rats. Chem. Biol. Interact. 2008;172(3):176–184. doi: 10.1016/j.cbi.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Zailanie K. Study of Padina australis using UV-VIS, HPLC and antibacterial. J. Life. Sci. Biomed. 2016;6(1):01–05. [Google Scholar]
- Zhang Y., He Y., Deng N., Chen Y., Huang J., Xie W. Protective effect of resveratrol against corticosterone-induced neurotoxicity in PC12 cells. Transl. Neurosci. 2019;10(1):235–240. doi: 10.1515/tnsci-2019-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.Y., Zhao Y.N., Zhong L.W., Huang Y.F. Chronic corticosterone exposure reduces hippocampal glycogen level and induces depression-like behavior in mice. J. Zhejiang Univ.-Sci. B. (Biomed. & Biotechnol.) 2015;16(1):62–69. doi: 10.1631/juz.B1400166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R., Jiang S., Zhang L., Yu Z. Mitochondrial electron transport chain, ROS generation and uncoupling (Review) Int. J. Mol. Med. 2019;44(1):3–15. doi: 10.3892/ijmm.2019.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Yin F., Jin G., Zhang X., Zhang L., Gong Z., Kang X., Hu H. In vitro neuroprotection of rat hippocampal neurons by manninotriose and astragaloside IV against corticosterone-induced toxicity. Molecules. 2018;23(12):E3339. doi: 10.3390/molecules23123339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B.H., Tan J., Zhang C., Wu Y. Neuroprotective effect of polysaccharides from Gastrodia elata Blume against corticosterone-induced apoptosis in PC12 cells via inhibition of the endoplasmic reticulum stress-mediated pathway. Mol. Med. Rep. 2018;17(1):1182–1190. doi: 10.3892/mmr.2017.7948. [DOI] [PubMed] [Google Scholar]
- Zhou Y.Z., Li X., Gong W.X., Tian J.S., Gao X.X., Gao L., Zhang X., Du G.H., Qin X.M. Protective effect of isoliquiritin against corticosterone-induced neurotoxicity in PC12 cells. Food Funct. 2017;8(3):1235–1244. doi: 10.1039/c6fo01503d. [DOI] [PubMed] [Google Scholar]