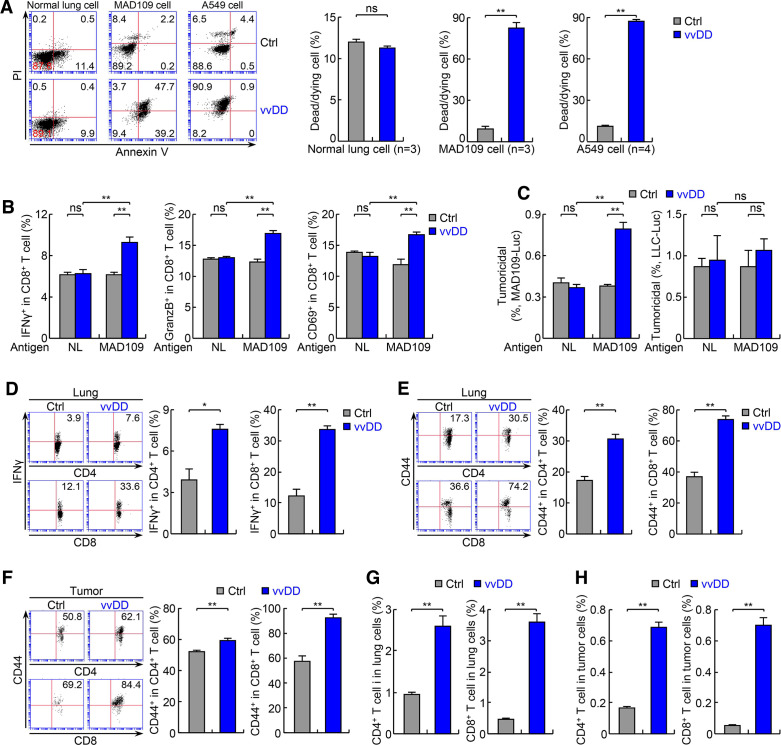
Figure 3.
The antitumor actions of vvDD involve direct oncolysis and indirect immune killing of tumor cells. (A) In vitro infection and FACS assays showing selective killing of mouse and human lung tumor cells but not normal cells by vvDD. (B) In vitro lung tumor antigen-dependent T-cell activation assays showing tumor-specific T-cell activation by vvDD (n=3). Normal lung cells: NL. (C) In vitro tumoricidal assay using T cells from (B) showing tumor antigen-dependent killing of T-cell specific MAD109 cells (left panel) but not T-cell non-specific LLC cells (right panel) (n=3). (D, E) FACS assays of IFNγ and CD44 showing increased lung CD4+ and CD8+ T-cell activation by vvDD treatment in urethane model (n=3). (F) FACS assay of CD44 showing increased activation of tumor-infiltrating CD4+ and CD8+ T cells by vvDD treatment in urethane model (n=4). (G) FACS assays showing increased lung CD4+ and CD8+ T cells by vvDD treatment in urethane model (n=6). (H) FACS assays showing increased tumor-infiltrating CD4+ and CD8+ T cells by vvDD treatment in urethane model (n=6). FACS, fluorescent activated cell sorting; IFNγ, interferon-γ; LLC, Lewis lung carcinoma; *p<0.05, **p<0.01; ns, not significant; vvDD, vaccinia virus with double deletion.