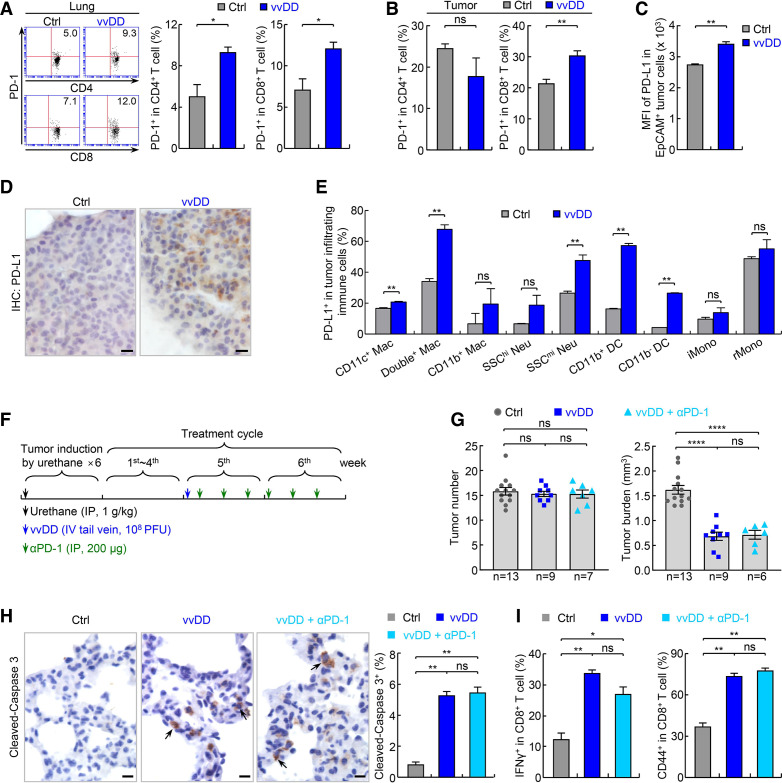
Figure 4.
PD-1 blockade fails to enhance vvDD’s efficacy in treating refractory lung cancer. (A) FACS assays showing increased pulmonary PD-1+ CD4+ and CD8+ T cells by vvDD treatment in urethane model (n=3). (B) FACS assays showing increased tumor-infiltrating PD-1+ CD8+ T cells by vvDD treatment in urethane model (n=5). (C) FACS assays showing increased PD-L1 expression on tumor cells by vvDD treatment in urethane model (n=3). (D) IHC assays showing increased PD-L1 expression on tumor cells by vvDD treatment in urethane model. scale bar, 10 µm. (E) FACS assays showing increased PD-L1 expression on tumor-associated immune cells by vvDD treatment in urethane model (n=3). (F) Tumor treatment schedule. (G) Urethane model showing no enhancement of vvDD efficacy in refractory lung cancer by PD-1 antibody. (H) IHC showing no enhancement of vvDD-induced tumor cell apoptosis by PD-1 antibody treatment in urethane model (n=9). Scale bar, 10 µm. (I) FACS assays of IFNγ and CD44 showing no significant increase in vvDD-induced CD8+ T-cell activation by PD-1 antibody treatment in urethane model (n=3). DC, dendritic cell; FACS, fluorescent activated cell sorting; IFNγ, interferon-γ; hi, high; iMono, inflammatory monocyte; IHC, immunohistochemistry; Mac, macrophage; mi, middle; Neu, neutrophil; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001; ns, not significant; PD-1, programmed cell death 1; PD-L1, programmed death ligand 1; rMono, resident monocyte; SSC, side scatter; vvDD, vaccinia virus with double deletion.