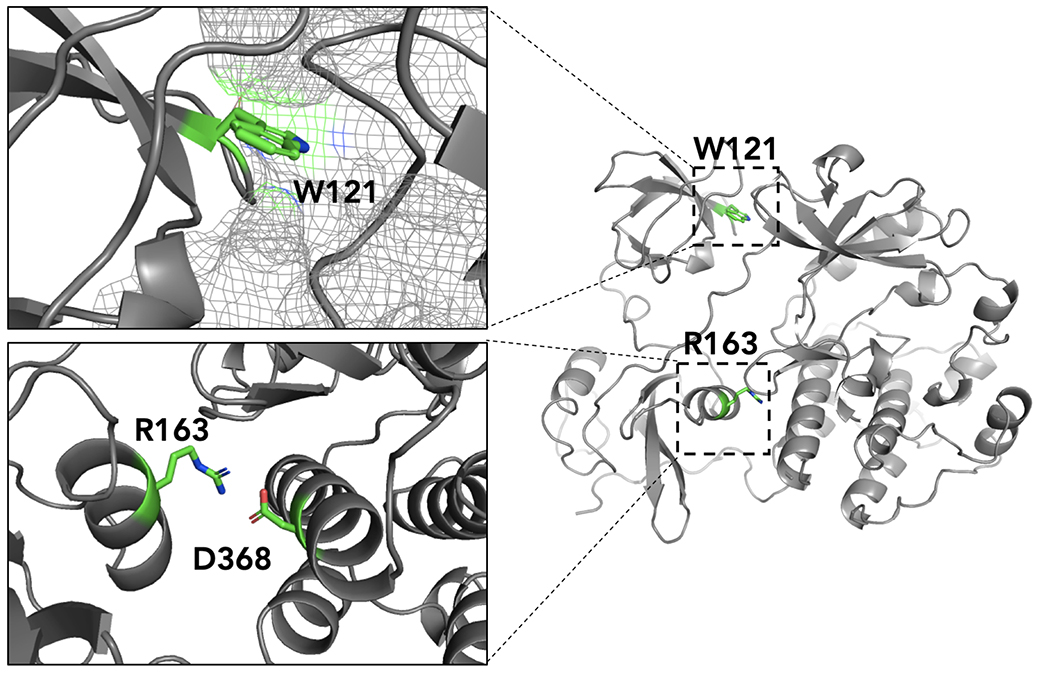
Figure 4. Trp-121 and Arg-163 are key residues that stabilize the closed conformation of c-Src.
Trp-121 is located in the SH3 domain and binds within a hydrophobic pocket on kinase domain. Arg-163 is located on the SH3 domain and forms a salt-bridge with Asp-368 that stabilizes the closed conformation. Mutations to these residues destabilize the closed conformation: W121R prevents Trp-121 binding within a hydrophobic pocket of the kinase domain; R163W disrupts the salt bridge between Arg-163 and Asp-368.