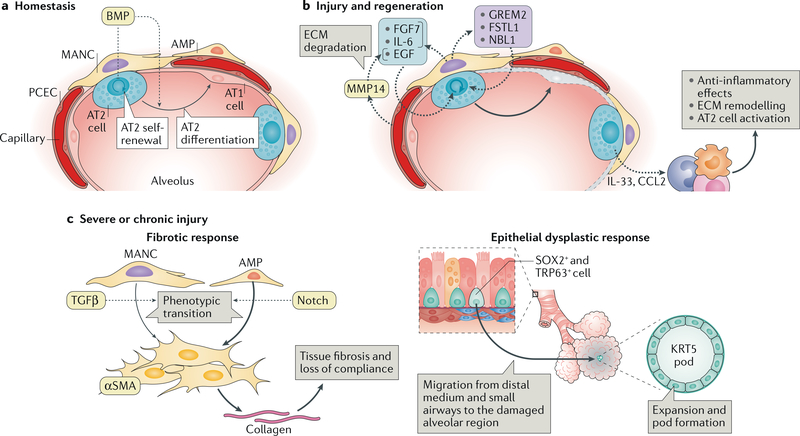
Fig. 5 |. Response of the alveolar niche to tissue injury.
a | The alveolar niche has two types of epithelial cell: alveolar type 1 (AT1) cells and alveolar type 2 (AT2) cells. The latter includes a WNT-responsive AT2 subset of cells that have been shown to serve as alveolar epithelial progenitors. At homeostasis the alveolar epithelial progenitors self-renew and differentiate into AT1 cells. These processes are regulated by bone morphogenetic protein (BMP) signalling, which restricts AT2 self-renewal, favouring AT1 differentiation. The rates of this homeostatic cell turnover are generally low. The stromal population comprises platelet-derived growth factor receptor- a-expressing fibroblasts, such as mesenchymal alveolar niche cells (MANCs), which are situated next to AT2 cells and Axin2-positive myofibrogenic precursors (AMPs), which are sparsely located throughout the alveoli and near blood vessels. b | In response to tissue injury, the alveolar niche upregulates ligands that promote AT2 cell proliferation and differentiation into AT1 cells (which are predominantly lost in response to insults; indicated in grey). Pulmonary capillary endothelial cells (PCECs) have been shown to respond to injury by upregulating matrix metalloproteinase 14 (MMP14), which by digesting the extracellular matrix (ECM) releases epidermal growth factor (EGF)-like ligands that stimulate epithelial cell growth. Injury- activated MANCs promote AT2 proliferation and self-renewal by producing FGF7 and IL-6 as well as BMP antagonists such as NBL1, GREM2 and FSTL1. AT2 cell-produced chemokines and cytokines, including CCL2 and IL-33, recruit monocytes (which differentiate into macrophages) and innate lymphoid cells (ILCs). Innate lymphoid cells support epithelial regeneration in part by dampening the acute inflammatory responses and by modulating the polarization of macrophages to the alternative (M2) lineage, which promotes AT2 cell proliferation likely via mechanisms involving ECM remodelling. c | Severe or chronic lung injury can result in dysplastic responses — fibrosis (left) and generation of abnormal cell clusters (right) — that if left unchecked will ultimately impair lung compliance and gas exchange. The fibrotic response is derived from fibroblasts — mostly AMPs and to a lesser extent MANCs — that proliferate on injury and transition into collagen-producing, alpha smooth muscle actin (αSMA)-positive myofibroblasts that deposit excessive amounts of ECM, which severely impairs lung compliance. A crucial signal for this myofibrogenic transition is provided by transforming growth factor-β(TGFβ), which is derived from the surrounding ECM. In the setting of severe alveolar epithelial cell destruction, such as that triggered by influenza infection, distally located SOX2-positive and TRP63-positive basal cells migrate out of the distal medium and/or small airways to regions of injury in the alveoli and expand as KRT5-expressing epithelial-like pods that radiate out of the tissue. The formation of these pods responds to hypoxia and is thought to be an epithelial cell-derived wound healing response that maintains the tissue barrier but ultimately does not generate functional alveolar epithelium.