Abstract
Genome-wide association studies (GWAS) have been performed for many psychiatric disorders and revealed a complex polygenic architecture linking mental and physical health phenotypes. Psychiatric diagnoses are often heterogeneous, and several layers of trait heterogeneity may contribute to detection of genetic risks per disorder or across multiple disorders. In this review, we discuss these heterogeneities and their consequences on the discovery of risk loci using large-scale genetic data. We primarily highlight the ways in which sex and diagnostic complexity contribute to risk locus discovery in schizophrenia, bipolar disorder, attention deficit hyperactivity disorder, autism spectrum disorder, posttraumatic stress disorder, major depressive disorder, obsessive-compulsive disorder, Tourette’s syndrome and chronic tic disorder, anxiety disorders, suicidality, feeding and eating disorders, and substance use disorders. Genetic data also have facilitated discovery of clinically relevant subphenotypes also described here. Collectively, GWAS of psychiatric disorders revealed that the understanding of heterogeneity, polygenicity, and pleiotropy is critical to translate genetic findings into treatment strategies.
Keywords: psychiatric disorders, heterogeneity, polygenicity, genome-wide association studies
Introduction
Genome-wide association studies (GWAS) are powerful tools for risk allele and gene discovery when applied to complex traits.1 The resulting data enable investigation of biological mechanisms, pathways, tissues, and cell types relevant for phenotype etiology,2–4 evolutionary pressures shaping genetic risk for a trait in the general population,5–9 and correlation and causal inference between traits.10–15
In psychiatry, GWAS have uncovered a high degree of polygenicity underlying mental illnesses and related complex phenotypes.8,16–35 Polygenicity describes the contribution of many single nucleotide polymorphisms (SNPs) with relatively small effect sizes to phenotype development. This phenomenon is ubiquitously observed in psychiatric disorders and comorbid phenotypes, as evidenced by the detection of tens to hundreds of genome-wide significant (GWS) linkage disequilibrium (LD) independent loci.1 Additionally, omnigenic models of complex traits suggest that highly interconnected gene regulatory networks influence trait etiology through a set of core genes and their associated regulatory elements and members of similar protein pathways.36
Though rare and structural variation contribute to the genetic liability of psychiatric disorders,37–39 the magnitude and ubiquity of GWAS data limit the scope of this review to common genetic variation. Here, we discuss GWAS-based methods for interrogating the etiology of psychiatric disorders. Next, we describe the polygenic nature of psychiatric disorders and how genetic and phenotype heterogeneity may affect our ability to detect risk loci for these traits. Finally, we briefly discuss future directions for the field of psychiatric genomics, including the advantage of large GWAS consortia and biobanks for exploring phenotype heterogeneity using genetic data.
GWAS for Detecting the Polygenic Architecture of Psychiatric Disorders
The primary goal of human genetics is to identify risk and protective factors for disease. Many aspects of human health and disease pose a challenge toward this goal. Complex traits lack a single gene with large enough effects to study in singularity with generalizable findings. Conversely, GWAS of complex traits have revealed large degrees of polygenicity underlying mental health (i.e. psychiatric disorders, behavior, personality traits, social science traits, and brain region measurements).1 GWAS are hypothesis-generating experiments that detect relationships between allele frequency and categorical or quantitative phenotypes.40,41 The results of GWAS are typically displayed as a Manhattan plot with base-pair positions ordered per chromosome on the x-axis and significance (–log10(association p value)) on the y-axis, creating a densely populated plot mimicking the Manhattan skyline.
A successful GWAS requires a well-considered phenotype. Three diagnostic classification systems for psychiatric disorders are used worldwide.42 The Diagnostic and Statistical Manual of Mental Disorders (DSM, currently the 5th edition) was developed by the American Psychiatric Association and is used primarily to guide clinical practice and mental health research. The International Classification of Diseases (ICD) was developed by the World Health Organization (WHO) and is used by clinicians for charting patient diagnosis of both physical and mental health conditions.43–45 The application of each system varies widely throughout the world, where DSM is widely used in the United States in contrast to the predominance of ICD in Europe.42 While there is a high degree of overlap between these systems, there is clinical heterogeneity, for example, in classifying some psychiatric outcomes.46,47 Phenotype heterogeneity may alter the sample allele distribution leading to (i) false negatives (i.e. true differences in allele frequency are masked) or (ii) false positives (i.e. allele frequency differences due to unbalanced phenotype distribution).48 The Research Domain Criteria (RDoC) paradigm compliments DSM and ICD classification systems by assessing clinical phenotypes hypothesized to more closely map onto underlying biological systems (e.g. neuroimaging data49 and brain circuit activity50). RDoC-based approaches offer an alternative to heterogeneous diagnostic systems, by permitting assessment of negative and positive valence, cognitive systems, sensorimotor systems, social processing systems, and/or arousal and regulatory systems across persons affected by different disorders, as well as healthy comparison subjects. Psychiatric disorders are often diagnosed based on a heterogeneous combination of symptom counts (i.e. an individual endorses a subset of symptoms but may not meet all criteria) as well as meeting full diagnostic criteria. These features also may be assessed for lifetime prevalence or, for example, last month prevalence. Note that herein we summarize findings from large studies of psychiatric disorders assessed with different instruments and considering different diagnostic criteria.
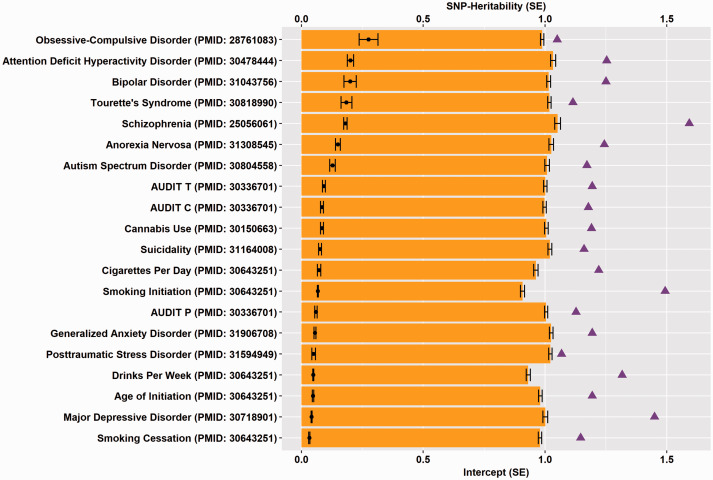
Large-scale GWAS have been used to understand many aspects of psychiatric disorders beyond risk locus detection. Analysis of GWAS after locus discovery is often termed “post-GWAS” analysis. Post-GWAS analyses are commonly used to follow-up risk locus discovery with additional sophisticated interpretation of GWAS signals. Some of these analyses are briefly described here. First, observed-scale heritability based on GWAS data (SNP-h2) reflects the contribution of common genetic information (rather than environmental or rare genetic factors) to the trait (Figure 1). This phenotype attribute may be conflated in case-control study designs by enrichment of cases relative to the general population prevalence. SNP-h2 also may be biased by residual population stratification from (i) higher than expected relatedness among samples or (ii) phenotype definition heterogeneity. It is understood that different functional classes of the genome disproportionately contribute to the SNP-h2. SNP-h2 estimates may then be partitioned to identify enrichment or depletion of certain functional classes of the genome such as enhancers, promoters, epigenetically regulated regions, and evolutionarily conserved regions. Another frequently-used post-GWAS method is genetic correlation whereby the per-SNP effects on one trait are regressed against the per-SNP effects of a second trait. The genetic liability for two traits may be positively, negatively, or not correlated. More sophisticated analytic tools also shed light on, or take advantage of, the polygenicity of psychiatric disorders, including (i) polygenic risk scoring (PRS, i.e. regressing weighted sum of per-SNP effects from one trait against another), (ii) Mendelian randomization (i.e. evaluating causality between phenotypes using genetic information as instrumental variables), (iii) functional annotation, fine-mapping, and co-localization to untangle polygenicity and prioritize casual risk loci, and (iv) structural equation modeling (i.e. identifying latent factors connecting phenotypes based on their genetic similarities).
Figure 1.
Test statistics from large-scale GWAS of psychiatric disorders (PMIDs provided) shed light on heritability and polygenicity. The orange bars represent the LDSC (linkage disequilibrium score regression) intercept, which indicates the presence of potential biases in the association analysis. The purple triangles represent the genomic inflection factor, which reflects the polygenicity of the trait when no inflation is present. The full black circles represent the SNP-Heritability. Where these statistics were not included in the associated manuscripts, they were calculated with LDSC using the 1000 Genomes Project European LD reference panel. For case-control phenotypes, SNP-h2 is plotted on the liability scale.
SNP: single nucleotide polymorphism.
Schizophrenia
Schizophrenia (SCZ) is a severe psychiatric disorder affecting 1% of the worldwide population.51 Due to the high morbidity and mortality, it is also known as the “cancer of mental illness.”52 SCZ is diagnosed based on “positive” and “negative” symptoms. The former include hallucinations and delusions, while the latter involves avolition and withdrawal.53,54 Additionally, SCZ cases often present with cognitive dysfunction and deficits in executive function.55 Twin- and family-based studies demonstrated that individuals related to SCZ cases have an increased lifetime disease risk, ranging from 50% for monozygotic twins to 2% for first cousins.56 Genome-wide studies based on high-throughput technologies have revolutionized our understanding of the genetic predisposition to SCZ. In 2014, the Psychiatric Genomics Consortium (PGC) conducted a GWAS in 36,989 SCZ cases and 113,075 controls,18 identifying 128 independent associations spanning 108 loci and proving that SCZ architecture is highly polygenic. In an updated PGC analysis including ∼60,000 SCZ cases,57 more than 250 GWS risk alleles have been identified, and an SCZ PRS showed that case-control group means differ by over 2/3 of a standard deviation (0.686; p = 1.1 × 10–254). Simulations of different degrees of polygenicity across complex traits showed that SCZ could be affected at least 20,000 causal loci.58 Among the SCZ-associated loci, the strongest association was observed in the major histocompatibility complex region.59 This appears to be due to structurally diverse alleles of the complement component 4 (C4) genes that lead to a greater expression of C4A in SCZ cases.60 Human C4 protein is localized to neuronal synapses, dendrites, and axons, and, in animal models, C4 appears to mediate synapse elimination during postnatal development.60 Although these previous findings support the role of increased synapse pruning in SCZ pathogenesis, a subsequent study in human postmortem brains showed that only the smallest dendritic spines are lost in deep layer 3 primary auditory cortex of SCZ, while larger dendritic spines are retained.61 While mechanistic studies are essential for understanding how genetic associations contribute to disease, genome-wide studies using polygenic instruments can shed light on genetic heterogeneity in relation to phenotypic heterogeneity across SCZ cases. Comparing SCZ and bipolar disorder (BD), it was possible to identify shared risk loci, as well as loci associated that distinguish the two disorders, and to characterize polygenic composition of multiple underlying symptom dimensions.16 Another SCZ characteristic is the negative association with cognitive ability and educational attainment.62 Genome-wide analyses showed that there is a positive genetic correlation between the liabilities to SCZ and educational attainment.63 This apparently “paradoxical” result is not due to possible confounders (e.g. LD or assortative mating) but suggests the presence of two potential SCZ subphenotypes: one resembling high intelligence and BD, while the other is a cognitive disorder that is independent of BD.64
Bipolar Disorder
BD is characterized by frequent mood swings between depressive and manic phases. The lifetime prevalence of BD is approximately 2.4% worldwide with twin-h2 of 80%.65 GWAS of BD have suggested a highly polygenic architecture so far comprising of 30 distinct loci associated with BD susceptibility.66 The most replicated risk genes are ankyrin 3 (ANK3) and calcium voltage-gated channel subunit alpha1 C (CACNA1C). Variants of these genes have been associated with white matter and total brain volume, thus implicating brain size as an intermediate phenotype. BD exists in two well-documented clinical subtypes: BD-I and BD-II.66,67 BD-I is distinguished from BD-II by extreme manic episodes experienced by those affected.68,69 The SNP-h2 of BD-I and BD-II have been estimated at 35% and 25%, respectively, with a 78% genetic overlap.70 This genetic overlap suggests many shared biological mechanisms contributing to each BD subtype but also suggests specific characteristics of the biological underpinnings of BD subtypes. For example, there was significantly greater relationship between (i) SCZ PRS and BD-I relative to BD-II and (ii) major depressive disorder (MDD) PRS and BD-II relative to BD-I.66,67,70–72 To date, the molecular mechanisms underlying these differences have yet to be robustly identified, but it is clear that studying BD as a single disorder may inflate heterogeneity and reduce power to detect the polygenic burden responsible for BD subtypes.
Attention Deficit Hyperactivity Disorder
Attention-deficit hyperactivity disorder (ADHD) is one of the most common psychiatric disorders affecting youths in the United States.73 ADHD is characterized by inability to focus, impulsivity, age-inappropriate hyperactivity, and increased rates of antisocial, anxiety, mood, and substance use disorders (SUDs). The lifetime ADHD prevalence ranges from 2% to 12%74 with twin-h2 estimates between 74% and 80%.75 A GWAS of 20,183 ADHD cases and 35,191 controls identified 12 GWS risk loci for ADHD and significant differences in SNP-h2 and risk locus detection between sexes.19,76–78 Males are two to seven times more likely to be diagnosed with ADHD than females and largely dominate the samples included in current GWAS.78,79 Several hypotheses have been proposed for this sex difference. First is the scenario that female ADHD is associated with a different set of variants as compared with male ADHD.80,81 Another hypothesis is that females are more resilient to developing ADHD and require a higher genetic burden to present relevant diagnostic symptoms.82,83 Though ADHD in males and females are highly genetically and phenotypically correlated,78,82 GWAS are only beginning to elucidate the differences in genetic liability to ADHD across sexes. So far, there is minimal evidence that polygenic risk for ADHD contributes to, or shares underlying biology with, different co-occurring conditions or behaviors unique to each sex.84 To date, there have been no GWS findings for ADHD in females though this may be attributed to decreased sample size and lower population prevalence. ADHD PRS has shown positive associations with educational and cognitive outcomes,85 body mass index,84 neuroticism,86 externalizing behaviors (e.g. smoking, aggression, impulsivity, risk-taking),82,84,85,87 and interpersonal communication behaviors.88 In addition to sex differences, ADHD is one of the most heterogeneously diagnosed psychiatric disorders with over 116,200 diagnostic combinations according to DSM-IV and DSM-5 criterion counts. Additionally, not all criteria are required to make an ADHD diagnosis such that two individuals with ADHD may not share any diagnostic criteria resulting in a level of diagnostic heterogeneity that may confound risk locus effects in genetic studies of ADHD.89 For example, the commonly implicated DAT1 underlying ADHD psychopathology has only robustly been implicated in ADHD cases without conduct-related diagnostic criteria.75,90
Autism Spectrum Disorder
Autism spectrum disorder (ASD) is the term used to describe a group of pervasive neurodevelopmental disorders characterized by impairment in social and communication skills often accompanied by repetitive and restrictive behaviors.23 Clinical manifestation of ASD is highly heterogeneous with the majority of ASD individuals receiving a diagnosis during early childhood and adolescence.91–95 Heterogeneity in ASD may manifest as intellectual capabilities ranging from severe disability to high intelligence quotients96 or the type of social cognition impaired (i.e. person-perceptive versus people-perceptive social skills).97,98 Clinically, specific ASD diagnoses tend to be defined based on the degree of intellectual ability in the affected. Asperger’s syndrome, for example, represents some of the least severe cognitive impairments along the autism spectrum.99,100 Because ASD manifests as a spectrum of phenotypes, grouping individuals into ASD cases versus controls may introduce heterogeneity even though cases cluster together on the primary phenotype level. It has been demonstrated that reducing this spectrum phenotype heterogeneity only modestly improves genetic homogeneity in contemporary studies with large ASD sample sizes.96 ASD affects 1% to 1.5% of the population, and males are diagnosed more often than females.101 Similar to ADHD, the hypothesis of a female protective effect exists for ASD whereby females may require a greater genetic burden to develop symptoms.101 There is evidence that testosterone levels of males relative to females may contribute to increased vulnerability to etiological factors in ASD cases102,103 and putatively defines mechanistic factors inducing heterogeneity detectable by large-scale genetic studies. These sex differences have recently been implicated in investigation strategies of empathizing-systemizing and automatizing-systemizing theories of ASD heterogeneity.97,98,104–106 The polygenic risk for ASD has been associated with cognitive ability,107 various changes in DNA methylation at birth,108 and gray matter volume in healthy and psychiatric patients.109 Studies of the relationship between genetic risk for ASD and other human health and disease phenotypes have revealed interesting findings. First, ASD genetic risk indeed predicts ASD severity; however, ASD PRS do not cleanly stratify individuals into more clinically severe ASD symptom criteria.107,110 This observation suggests that though phenotypic ASD subtypes exist, they may not appropriately stratify ASD for genetic studies.96 Second, the genetic risk for ASD in ASD unaffected individuals (i.e. unaffected individuals carrying ASD) associates with features of healthy neurodevelopment.98,106
Posttraumatic Stress Disorder
Posttraumatic stress disorder (PTSD) affects individuals who have experienced, witnessed, or been confronted with an event involving actual or threatened danger. This required environmental component of PTSD makes it unique among DSM-5 disorders without such required etiologies. Diversity among traumatic experience adds substantial heterogeneity to PTSD cases, and this diversity can be detected with GWAS.27,30 Given the abundance of PTSD in veteran populations, exposure to combat-related experiences has garnered much attention. Two large studies of PTSD estimated SNP-h2 of 2% to 5% in the PGC international meta-analysis and 6.4% to 10.1% in the Million Veteran Program (MVP).30 These cohorts represent different trauma exposures with MVP comprised mostly of males exposed to military combat and PGC comprised of international sex-balanced PTSD cases exposed primarily to civilian traumas. Traumatic events vary by sex, demography, and socioeconomic status. Males have higher rates of overall trauma exposure, yet females are more likely to develop PTSD following similar trauma, resulting in approximately doubled lifetime PTSD prevalence in U.S. females (8%) relative to males (4.1%).111,112 Furthermore, sexual trauma is more prevalent among females, and males are more likely to experience nonsexual assaults, death/injury, and military combat. Specific traumas also appear to convey different magnitudes of risk for PTSD.113 Sex differences are reflected in sex-stratified GWAS where the SNP-h2 of PTSD in males was no different from zero regardless of ancestry and the SNP-h2 of PTSD in females was 8% to 18% with African ancestry individuals demonstrating the highest SNP-h2 estimates.27,114 Additional heterogeneity of PTSD can be seen among the responses to trauma evaluated for PTSD diagnosis.22,30 Including the PTSD symptom criteria of reexperiencing, avoidance, negative emotional symptoms, and hyperarousal, there are 636,120 possible combinations by which a person may be diagnosed with PTSD.115 Several loci (localizing near genes MAD1L1, TCF4, and TSNARE1) have been implicated across symptoms, lending support for these loci as putative targets for PTSD treatment. Lastly, there is considerable heterogeneity in the longitudinal course of PTSD, with distinct trajectories of symptom onset, severity, and remission that may be in part related to trauma context, sex, access to care, and other unmeasured influences.116,117 Future work in PTSD genetics will surely begin addressing how differences in genetic risk also influence PTSD trajectory and prognosis.
Major Depressive Disorder
MDD is the unceasing depressive or low mood lasting for more than two weeks accompanied by disturbances in weight, circadian rhythms, elevated negative emotions, and self-debilitating thoughts. The lifetime prevalence of MDD in the United States is approximately 20.6%,118 and twin-h2 is estimated between 30% and 40%.119 MDD is associated with socioeconomic burden and all-cause mortality and is thus a leading cause of disease burden worldwide.120 Phenotypic heterogeneity of MDD is primarily driven by sex differences; the lifetime prevalence of MDD in females is 26.1% and in males is 14.7%.118 To date, GWAS have identified many loci conferring risk for MDD, the largest of which (N = 807,553 individuals) detected 102 MDD risk loci.24,32,121–123 Though effective sample sizes continue to increase, SNP-h2 estimates converge between 8.7% and 8.9%.24 Heterogeneity in MDD may be evident by the presence of five DSM-IV diagnostic criteria and lower SNP-h2 estimates in males versus females.124 Furthermore, many of the single-item criteria for an MDD diagnosis (e.g. “nerves, anxiety, tension or depression,” and “self-reported depressive symptoms with associated impairment” items in the UK biobank) overlap with multi-item diagnostic instruments for other disorders such as BD and SCZ.24,121,125 These shared diagnostic criteria may reduce accuracy of PRS in stratifying MDD individuals and introducing heterogeneous genetic associations. It is important to note that MDD, like other psychiatric disorders, exists as a spectrum rather than clear case-control distinction, and the reduction of MDD into such a binary classification system may introduce heterogeneity among case and control categories.
Obsessive-Compulsive Disorder
Obsessive-compulsive disorder (OCD) is characterized by recurrent, intrusive, or unwanted thoughts, images, or impulses that provoke anxiety and actions to ameliorate that anxiety. OCD cases display concerns about contamination, responsibility for harm or injury, unacceptable thoughts that are often sexual and/or religious in nature, and symmetry, completeness, and the need for things to be “just right.” Compulsive behaviors to neutralize anxiety include excessive cleaning/hygiene, repeated checking, or other ritualized thoughts and behaviors. The lifetime prevalence of OCD is 1% to 3%,126,127 and twin-h2 is estimated to be 48%.128 To date, OCD GWAS have not detected any GWS risk loci, but genetic data estimate SNP-h2 at 28%.129 There are notable sex differences observed in clinical presentations of OCD, with males comprising roughly two thirds of childhood-onset cases and reporting a higher incidence of obsessions related to religious/sexual thoughts and symmetry themes.127,130 Females are more likely to present with late-onset OCD and report higher rates of precipitating events (e.g. pregnancy and childbirth), exacerbation of symptoms with hormonal events, and higher rates of comorbid eating disorder.127,130 Obsessive themes among females with OCD tend to center around hygiene.127 Large-scale GWAS of OCD in sex-stratified cohorts failed to detect significant SNP-h2 in males but estimate significant SNP-h2 in females (30%). Sex-stratified OCD GWAS are underpowered to formally test the genetic correlation between them; however, per-SNP effect sizes tend to positively associate. One study suggested that SNP-h2 may vary across different age groups with greater SNP-h2 estimates in OCD of younger individuals (h2=0.43) versus older individuals (h2 not significant).131 OCD has received considerable attention due to its relationship with other psychiatric disorders. In a familial co-aggregation study, first-degree relatives of OCD patients had more than double the risk for BD (relative risk (RR) confidence interval (CI) = 2.68–3.04), MDD (RR CI = 2.58–2.67), ASD (RR CI = 2.10–2.71), ADHD (RR CI = 2.07–2.32), and SCZ (RR CI = 1.86–2.09).132 Exploring how OCD relates to these other psychiatric disorder revealed overlapping exomes and polygenic risk between OCD and SCZ and identified DMN3 as one suggestive link between the two disorders.133
Tourette’s Syndrome and Chronic Tic Disorder
Tourette’s syndrome (TS) is typically diagnosed before 18 years of age and requires two or more motor and at least one phonic tic lasting more than one year.134 The related diagnosis, chronic tic disorder (CTD), requires the presence of two or more of either motor or phonic tics, but not both. TS has a prevalence of 0.3% to 0.8% and occurs more frequently in males, at a ratio of approximately 3.5:1,135 with similar prevalence reported for CTD.136,137 Though males tend to be diagnosed more often than females, females experience greater day-to-day burden of more severe tics.138–140 The largest GWAS of TS (N = 14,307) identified a single GWS variant and 39 suggestive associations.33 Estimates of TS SNP-h2 range from 21% to 58%,33,131 with twin-h2 estimates up to 60%.131 TS PRS were predictive of clinical status in independent samples, with probands from multiplex families showing higher loading than those from simplex families.33,141 Individuals with CTD have elevated TS PRS relative to controls. Childhood neurodevelopmental disorders (e.g. ADHD, ASD, OCD, and TS/CTD) share elevated rates of comorbidity as well as shared subphenotypes (e.g. executive functioning, impulse control, intrusive thoughts, repetitive behaviors, and rigid adherence to routines), which pose challenges for both clinical subphenotyping and for understanding genetic effects. TS PRS have been associated with (i) the presence, but not chronicity of tics and (ii) the severity of symptoms associated with comorbid conditions.142 Much of the heterogeneity associated with TS and CTD stems from the relationship between TS/CTD and OCD and ADHD. Two TS subphenotypes have been detected, the symmetry subphenotype and disinhibition subphenotype, which have distinct genetic architectures not fully understood due to strict adherence to DSM-based diagnoses.143 The symmetry subphenotype was positively predicted by TS (but not OCD or ADHD) PRS, while the disinhibition subphenotype was predicted by OCD (but no other) PRS. In a cross-trait gene-based study of OCD and TS, CADM2, LY6G6F, MEGT1, and APOM were identified as GWS loci but were not detected in other pairwise neurodevelopmental disorder gene-based analyses142 To disentangle and specify genetic relationships among these disorders, future studies may benefit from (i) the incorporation of shared intermediate phenotypes (ii) post hoc conditioning of analyses to remove shared or nonspecific effects, and (iii) investigating molecular mechanism involving identified gene targets shared and differentially expressed between TS and other psychiatric disorders (e.g. OCD).143
Anxiety Disorders
Anxiety disorders in DSM-5 include separation anxiety, selective mutism, specific phobias, panic disorder, agoraphobia, and generalized anxiety disorder (GAD). The lifetime prevalence of anxiety is estimated at 31%.144,145 Twin studies suggest anxiety disorder twin-h2 of 42%.146 Anxiety disorders are quite heterogeneous, and many subtypes are ubiquitously more prevalent in females.147 GAD is perhaps the most thoroughly investigated by large-scale GWAS. GAD is defined as the presence of excessive anxiety and worry about a variety of topics, events, or activities, typically lasting more than six months. The excessive worry associated with GAD is often to the point where the affected cannot control themselves. Physical symptomology often varies between cases and may include edginess, impaired concentration, empty mindedness, irritability, muscle aches, and sleeplessness.148–150 Recent GWAS of GAD in >200,000 U.S. military veterans identified risk loci shared between GAD and SCZ and BD.25 Collectively, the polygenic architecture of GAD-2 (a two-item GAD symptom criterion checklist) significantly overlapped with MDD, neuroticism, and PTSD.25 Though significant overlap exists between various measures of anxiety disorders (anxiety case-control,151 GAD-2,25 and GAD-7152), these overlaps are not perfect, suggesting that the distribution of anxiety symptoms in study cohorts may be readily detected in the associated genetic data. Anxiety disorders demonstrate substantial heterogeneity based on age of the ascertained cohort.153,154 Two anxiety disorders, separation anxiety and selective mutism, were once thought to be exclusively childhood disorders, but it is now accepted that children and adults may receive these diagnoses. There also is evidence that environmental heterogeneity, such as childhood maltreatment, moderate polygenic risk in genetic studies of anxiety disorders.155–157
Suicidality
Worldwide, more than 1 million people complete suicide every year making it the 10th leading cause of death in the United States (12.6 deaths per 100,000).158 Nonfatal suicidal behaviors also are a consistent emotional and economic burden. Suicidal ideation, plans, gestures, attempts, and completed suicides represent a continuum of suicidal behavior. There is one death by suicide for every 25 attempts,159 and some of attempts are severe enough to require medical attention and may have long-lasting sequelae. Having a psychiatric disorder is a major risk factor for suicidal behaviors and individuals affected by a mental illness may represent at least 90% of the people who have died by suicide.160 However, most people with mental disorders do not die by suicide, and the risk of suicide is 5% to 8% for several mental disorders.161 According to the stress-diathesis model, the risk for suicidal acts is determined a stressor (such as psychiatric illness) and a diathesis, such as a tendency to experience more suicidal ideation and be more likely to act on suicidal feelings.162 Twin, family, and adoption studies identified a 30% to 50% h2 which appears to be partially independent from psychiatric disorders.163 GWAS have just started to investigate suicidal behaviors in relatively large cohorts.67,164–167 These analyses were conducted on cohorts with different characteristics including general population, military personnel, and individuals affected by psychiatric disorders. Although few risk loci were identified, a consistent genetic overlap has been observed between MDD and suicidal behaviors.67,164–167 A recent multivariate genome-wide interaction study detected that genetic risk for suicidal behaviors is partially moderated by multivariate gene interactions linking comorbid substance dependences with suicidal ideation.168 This suggests that the phenotypic heterogeneity among individuals experiencing suicidal behaviors increases the genetic complexity of these traits. Genome-wide approaches have the potential to disentangle the diverse characteristics observed among individuals experiencing suicidal feelings and committing a suicidal attempt. However, much more informative cohorts are needed to achieve a comprehensive understanding of the molecular basis of suicidal behaviors.
Feeding and Eating Disorders
Eating disorders are classified as abnormal eating episodes occurring intermittently or frequently and lasting for more than three months. DSM-IV recognized three primary diagnoses: anorexia nervosa (AN), bulimia nervosa (BN), and eating disorders not otherwise specified (called EDNOS; diagnosed by omission of symptom criteria for AN and BN). The prevalence rates of eating disorders are affected by socioeconomic status. Females experience higher prevalence than men, non-Hispanic populations of European descent tend to have higher prevalence than individuals of other ethnicities, and there is some evidence that family income may contribute to eating disorder prevalence.169 Psychiatric comorbidities are common among eating disorder cases, including MDD, anxiety, and OCD.170 The twin-h2 of eating disorders varies from 40% to 60%.171 The SNP-h2 of eating disorders (cases included AN and BN; N∼14,000) was 20% in early studies of this class of disorders.172
Anorexia Nervosa
AN has a prevalence of 0.8% and is characterized by restricted eating, weight loss, difficulties maintaining an age- or height-appropriate body weight, and distorted body image. Genetic studies have mostly focused on AN likely due to the higher population prevalence compared to other eating disorders. The most recent and largest GWAS of 76,644 individuals detected eight risk loci using diagnostic criteria from DSM-III to DSM-5. The study reported SNP-h2 of approximately 11% and relatively high genomic lambda (1.22) but appropriate LD intercept which collectively provide evidence of high polygenicity. Partitioning the SNP-h2 for mouse model cell types identified spiny and pyramidal neurons of the hippocampus, which are responsible for feeding behavior and impetus. Additionally, reported genetic correlation of AN with psychiatric disorders and metabolic dysregulation parallels epidemiologically observed comorbidities.31
Bulimia Nervosa
BN is characterized by cycles of bingeing and caloric compensatory behaviors, such a vomiting, and its prevalence is 0.28%.169 While GWAS have provided much needed resolution of genetic liability to AN, the genetic liabilities of BN, and other eating disorders are less clear. Larger sample sizes will indeed be required to detect genetic correlations, SNP-h2, and underlying biology associated with BN such that therapeutic and diagnostic interventions may be developed.171
Substance Use Disorders
SUDs are characterized by uncontrolled desire for excessive substance intake and inability to reduce the frequency of consumption. According to the WHO, there are more than 180-million drug users worldwide.173 DSM-5 has expanded SUD definition to include gambling disorders and combines the concepts of substance abuse and dependence, though there is evidence of shared and specific genetic effects for these traits.174 Commonly studied substances include alcohol, stimulants (e.g. amphetamines and cocaine), tobacco, and opioids.175 Phenotypic heterogeneity in SUDs stems from various drug seeking patterns, environmental factors, pharmacokinetic and pharmacodynamic processes, and psychiatric comorbidities.173 The characterization of behavioral and psychiatric traits related to the use and abuse of addictive substances in large cohorts remain challenging due to several factors, such as the hypothesized differences in recreational versus prescription use and abuse and the societal stigma associated with use of certain substances over others. These barriers are being overcome through large consortium and biobank efforts, but studies of some substances (e.g. opioids and cocaine) remain underpowered to make robust conclusions about underlying biology.
Alcohol
Individuals with alcohol use disorder (AUD) had high comorbidity with other psychiatric disorders, while alcohol consumption has a much lower genetic correlation with psychiatric disorders.176 AUD is measured by dependence on extreme alcohol consumption and has a twin-h2 of 50%.177 SNP-h2 for alcohol dependence averages around 10%. GWAS for alcohol dependence using DSM and alcohol consumption with the AUD Identification Test converge on variants in the alcohol metabolizing gene ADH1B and other genes (GCKR, SLC39A8, FTO, ADH4, SIX3, and DRD2) with shared biological functionality. Only half of the genes overlap between alcohol consumption and AUD suggesting distinct etiologies between consuming/using alcohol and being dependent on its effects.178
Nicotine
Smoking cigarettes, whose primary substance is nicotine, is a complex phenotype ranging from initiation, consistent pattern, dependence, termination, and reversion. The family-h2 for nicotine dependence is measured to be 75%.179 GWAS have consistently replicated a region on chromosome 15 consisting of CHRNA3, CHRNA4, and CHRNA5 which explain 4% to 5% of the variance in smoking-related phenotypes. These results are analogous to other SUDs, drawing attention to biological heterogeneity varying with the severity of dependence.174,180,181
Opioids
Like AUDs, assessing differences between opioid use and dependence proves essential to understanding the polygenic architectures of these traits.20 A recent study comparing opioid-exposed versus unexposed controls detected SNP-h2 of 28%.174 A study conducted in 10,544 OUD cases and 72,163 opioid-exposed controls from the MVP cohort identified OPRM1 Asn40Asp (rs1799971) as a significant risk locus, also showing genetic correlation with multiple substance use traits and psychiatric illnesses and possible causal effects on OUD risk from tobacco smoking, major depression, neuroticism, and cognitive performance.34
Cannabis
The family-wise h2 of cannabis use is 45%, and recent GWAS of 184,765 individuals reported SNP-h2 of 11%. Genetic risk for cannabis use was positively genetically correlated with MDD and SCZ, risk-taking behavior, and neuroticism.182 Age at cannabis initiation also appears to be moderately heritable, and the significant association with ATP2C2 is consistent with the role of calcium signaling mechanisms in the propensity to cannabis use.183 In a GWAS of cannabis dependence, there is a consistent overlap with potential genetic factors contributing to major depression and SCZ.184
Conclusions and Future Directions
GWAS have contributed major advances to our understanding of the polygenic architecture of psychiatric disorders. However, the phenotypic and genetic heterogeneity described herein contribute to complicate the translation of genetic data into clinical practice. For example, sex differences are ubiquitous across psychiatric disorders but are only recently being investigated with genome-wide methods. Unfortunately, stratifying by sex drastically reduces sample size for a GWAS, but large-scale genomics consortia are rapidly collecting suitable sample sizes to make these analyses more feasible and reliable. Until then, the community may consider focusing attention on several additional sex-specific topic areas including X-chromosome studies and regulatory/expression studies of risk loci. X-chromosome association studies are still relatively novel and require additional consideration of dosage differences between sexes but hold great potential for uncovering differential disorder risks in males and females.185–187 Furthermore, regulatory mechanisms have been identified as likely contributors to the sex differences in many disorders, but these processes remain vastly underinvestigated.188–190 These studies will be particularly informative for understanding how genes discovered by GWAS are expressed in each sex.
We have summarized how phenotypic heterogeneity greatly influences genetic heterogeneity in psychiatry. The RDoC paradigm may help considerably reduce this heterogeneity by focusing on biologically tractable processes, measurements, and/or mechanisms rather than diagnoses dependent on multi-item symptom checklists.49 It is important to note that some of the existing GWAS of psychiatric disorders likely already incorporate some aspects of RDoC (e.g. (a) hyperarousal and reexperiencing symptoms of PTSD diagnosis22 and (b) studying hallucinations as a representative symptom of psychotic disorders191).
GWAS of psychiatric disorders have elucidated thousands of risk loci contributing to disease etiology and generated countless testable hypothesis addressing psychiatric disorder heterogeneity, comorbidities, and cross-species interactions (e.g. microbiome-brain interactions). GWAS data, and therefore the resulting post-GWAS analyses, may be influenced by phenotype and sample heterogeneity which both have document effects on detection of the polygenic architecture of a trait. It is well understood that the polygenicity of a disorder in one population may not reflect the polygenicity of the same disorder in an external population. This observation means that findings from well-studied European populations may not, and indeed do not,192 translate effectively to non-Europeans and supports a discipline-wide effort to close this gap by studying psychiatric disorder polygenic architectures in other populations.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors are supported by the National Institute on Drug Abuse (R21 DA047527), the National Institute National Institute on Deafness and Other Communication Disorders (R21 DC018098), and the National Center for Posttraumatic Stress Disorder of the U.S. Department of Veterans Affairs.
ORCID iDs
Frank R. Wendt https://orcid.org/0000-0002-2108-6822
Daniel S. Tylee https://orcid.org/0000-0002-7579-6096
Renato Polimanti https://orcid.org/0000-0003-0745-6046
References
- 1.Buniello A, MacArthur JAL, Cerezo M, et al. The NHGRI-EBI GWAS catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019; 47: D1005–D1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akbarian S, Liu C, Knowles JA, et al. The PsychENCODE project. Nat Neurosci. 2015; 18: 1707–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watanabe K, Taskesen E, van Bochoven A, Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat Commun. 2017; 8: 1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watanabe K, Umicevic Mirkov M, de Leeuw CA, van den Heuvel MP, Posthuma D. Genetic mapping of cell type specificity for complex traits. Nat Commun. 2019; 10: 3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huber CD, DeGiorgio M, Hellmann I, Nielsen R. Detecting recent selective sweeps while controlling for mutation rate and background selection. Mol Ecol. 2016; 25: 142–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hujoel MLA, Gazal S, Hormozdiari F, van de Geijn B, Price AL. Disease heritability enrichment of regulatory elements is concentrated in elements with ancient sequence age and conserved function across species. Am J Hum Genet. 2019; 104: 611–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Connor LJ, Schoech AP, Hormozdiari F, Gazal S, Patterson N, Price AL. Extreme polygenicity of complex traits is explained by negative selection. Am J Hum Genet. 2019; 105: 456–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pardinas AF, Holmans P, Pocklington AJ, et al. Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat Genet. 2018; 50: 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polimanti R, Gelernter J. Widespread signatures of positive selection in common risk alleles associated to autism spectrum disorder. PLoS Genet. 2017; 13: e1006618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med. 2017; 36: 1783–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulik-Sullivan B, Finucane HK, Anttila V, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015; 47: 1236–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bulik-Sullivan BK, Loh PR, Finucane HK, et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015; 47: 291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebrahim S, Davey Smith G. Mendelian randomization: can genetic epidemiology help redress the failures of observational epidemiology? Hum Genet. 2008; 123: 15–33. [DOI] [PubMed] [Google Scholar]
- 14.O’Connor LJ, Price AL. Distinguishing genetic correlation from causation across 52 diseases and complex traits. Nat Genet 2018; 50: 1728–1734. [DOI] [PMC free article] [PubMed]
- 15.Wendt FR, Pathak GA, Lencz T, Krystal JH, Gelernter J, Polimanti R. Dissecting the genetic overlap of education, socioeconomic status, and mental health. medRxiv 2020; 2020.01.09.20017079.
- 16.Bipolar Disorder and Schizophrenia Working Group of the Psychiatric Genomics Consortium. Genomic dissection of bipolar disorder and schizophrenia, including 28 subphenotypes. Cell. 2018; 173: 1705–1715.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cross-Disorder Group of the Psychiatric Genomics Consortium. Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell. 2019; 179: 1469–1482.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014; 511: 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demontis D, Walters RK, Martin J, et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet. 2019; 51: 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gelernter J, Kranzler HR, Sherva R, et al. Genome-wide association study of opioid dependence: multiple associations mapped to calcium and potassium pathways. Biol Psychiatry. 2014; 76: 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gelernter J, Sherva R, Koesterer R, et al. Genome-wide association study of cocaine dependence and related traits: FAM53B identified as a risk gene. Mol Psychiatry. 2014; 19: 717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gelernter J, Sun N, Polimanti R, et al. Genome-wide association study of post-traumatic stress disorder reexperiencing symptoms in >165,000 US veterans. Nat Neurosci. 2019; 22: 1394–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grove J, Ripke S, Als TD, et al. Identification of common genetic risk variants for autism spectrum disorder. Nat Genet. 2019; 51: 431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howard DM, Adams MJ, Clarke TK, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. 2019; 22: 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey DF, Gelernter J, Polimanti R, et al. Reproducible genetic risk loci for anxiety: results from approximately 200,000 participants in the million veteran program. Am J Psychiatry. 2020; 177: 223–232. [DOI] [PMC free article] [PubMed]
- 26.Liu M, Jiang Y, Wedow R, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. 2019; 51: 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nievergelt CM, Maihofer AX, Klengel T, et al. International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat Commun. 2019; 10: 4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pasman JA, Verweij KJH, Gerring Z, et al. GWAS of lifetime cannabis use reveals new risk loci, genetic overlap with psychiatric traits, and a causal influence of schizophrenia. Nat Neurosci. 2018; 21: 1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polimanti R, Walters RK, Johnson EC, et al. Leveraging genome-wide data to investigate differences between opioid use vs. opioid dependence in 41,176 individuals from the Psychiatric Genomics Consortium. bioRxiv 2019; 765065. [DOI] [PMC free article] [PubMed]
- 30.Stein MB, Levey DF, Cheng Z, et al. Genomic characterization of posttraumatic stress disorder in a large US military veteran sample. bioRxiv 2019; 764001.
- 31.Watson HJ, Yilmaz Z, Thornton LM, et al. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat Genet. 2019; 51: 1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wray NR, Ripke S, Mattheisen M, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018; 50: 668–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu D, Sul JH, Tsetsos F, et al. Interrogating the genetic determinants of Tourette’s syndrome and other tic disorders through genome-wide association studies. Am J Psychiatry. 2019; 176: 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou H, Rentsch CT, Cheng Z, et al. GWAS including 82,707 subjects identifies functional coding variant in OPRM1 gene associated with opioid use disorder. medRxiv. 2019; 19007039.
- 35.Zhou H, Sealock JM, Sanchez-Roige S, et al. Meta-analysis of problematic alcohol use in 435,563 individuals identifies 29 risk variants and yields insights into biology, pleiotropy and causality. bioRxiv 2019; 738088. [DOI] [PMC free article] [PubMed]
- 36.Boyle EA, Li YI, Pritchard JK. An expanded view of complex traits: from polygenic to omnigenic. Cell. 2017; 169: 1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bis JC, Jian X, Kunkle BW, et al. Whole exome sequencing study identifies novel rare and common Alzheimer’s-associated variants involved in immune response and transcriptional regulation. Mol Psychiatry 2018. [DOI] [PMC free article] [PubMed]
- 38.Weiner DJ, Wigdor EM, Ripke S, et al. Polygenic transmission disequilibrium confirms that common and rare variation act additively to create risk for autism spectrum disorders. Nat Genet. 2017; 49: 978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu C, Arcos-Burgos M, Baune BT, et al. Low-frequency and rare variants may contribute to elucidate the genetics of major depressive disorder. Transl Psychiatry. 2018; 8: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lam M, Awasthi S, Watson HJ, et al. RICOPILI: Rapid Imputation for COnsortias PIpeLIne. Bioinformatics. 2020; 36: 930–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marees AT, de Kluiver H, Stringer S, et al. A tutorial on conducting genome-wide association studies: quality control and statistical analysis. Int J Methods Psychiatr Res. 2018; 27: e1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clark LA, Cuthbert B, Lewis-Fernandez R, Narrow WE, Reed GM. Three approaches to understanding and classifying mental disorder: ICD-11, DSM-5, and the National Institute of Mental Health’s Research Domain Criteria (RDoC). Psychol Sci Public Interest. 2017; 18: 72–145. [DOI] [PubMed] [Google Scholar]
- 43.Cartwright DJ. ICD-9-CM to ICD-10-CM codes: What? Why? How? Adv Wound Care (New Rochelle). 2013; 2: 588–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Malley KJ, Cook KF, Price MD, Wildes KR, Hurdle JF, Ashton CM. Measuring diagnoses: ICD code accuracy. Health Serv Res. 2005; 40: 1620–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Regier DA, Kuhl EA, Kupfer DJ. The DSM-5: classification and criteria changes. World Psychiatry. 2013; 12: 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adornetto C, Suppiger A, In-Albon T, Neuschwander M, Schneider S. Concordances and discrepancies between ICD-10 and DSM-IV criteria for anxiety disorders in childhood and adolescence. Child Adolesc Psychiatry Ment Health. 2012; 6: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suris A, Holliday R, North CS. The evolution of the classification of psychiatric disorders. Behav Sci (Basel). 2016; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wendt FR, Pathak GA, Overstreet C, et al. Natural selection influenced the genetic architecture of brain structure, behavioral and neuropsychiatric traits. bioRxiv 2020; 2020.02.26.966531.
- 49.Montalvo-Ortiz JL, Gelernter J, Hudziak J, Kaufman J. RDoC and translational perspectives on the genetics of trauma-related psychiatric disorders. Am J Med Genet B Neuropsychiatr Genet. 2016; 171B: 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nicholson JR, Sommer B. The research domain criteria framework in drug discovery for neuropsychiatric diseases: focus on negative valence. Brain Neurosci Adv. 2018; 2: 2398212818804030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Birnbaum R, Weinberger DR. Genetic insights into the neurodevelopmental origins of schizophrenia. Nat Rev Neurosci. 2017; 18: 727–740. [DOI] [PubMed] [Google Scholar]
- 52.Jablensky A, Kirkbride J, Jones PB. Schizophrenia: the epidemiological horizon. In: Weinberger DR and Harrison PJ, eds. Schizophrenia Wiley-Blackwell; 2011: 185–225.
- 53.Marder SR, Galderisi S. The current conceptualization of negative symptoms in schizophrenia. World Psychiatry. 2017; 16: 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel KR, Cherian J, Gohil K, Atkinson D. Schizophrenia: overview and treatment options. P T. 2014; 39: 638–645. [PMC free article] [PubMed] [Google Scholar]
- 55.Orellana G, Slachevsky A. Executive functioning in schizophrenia. Front Psychiatry. 2013; 4: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin AR, Daly MJ, Robinson EB, Hyman SE, Neale BM. Predicting polygenic risk of psychiatric disorders. Biol Psychiatry. 2019; 86: 97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sullivan PF, Geschwind DH. Defining the genetic, genomic, cellular, and diagnostic architectures of psychiatric disorders. Cell. 2019; 177: 162–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loh PR, Bhatia G, Gusev A, et al. Contrasting genetic architectures of schizophrenia and other complex diseases using fast variance-components analysis. Nat Genet. 2015; 47: 1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mokhtari R, Lachman HM. The major histocompatibility complex (MHC) in schizophrenia: a review. J Clin Cell Immunol. 2016; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sekar A, Bialas AR, de Rivera H, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016; 530: 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.MacDonald ML, Alhassan J, Newman JT, et al. Selective loss of smaller spines in schizophrenia. Am J Psychiatry. 2017; 174: 586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tripathi A, Kar SK, Shukla R. Cognitive deficits in schizophrenia: understanding the biological correlates and remediation strategies. Clin Psychopharmacol Neurosci. 2018; 16: 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ohi K, Sumiyoshi C, Fujino H, et al. Genetic overlap between general cognitive function and schizophrenia: a review of cognitive GWASs. Int J Mol Sci. 2018; 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bansal V, Mitjans M, Burik CAP, et al. Genome-wide association study results for educational attainment aid in identifying genetic heterogeneity of schizophrenia. Nat Commun. 2018; 9: 3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Craddock N, Sklar P. Genetics of bipolar disorder: successful start to a long journey. Trends Genet. 2009; 25: 99–105. [DOI] [PubMed] [Google Scholar]
- 66.Stahl EA, Breen G, Forstner AJ, et al. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat Genet. 2019; 51: 793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stein MB, Ware EB, Mitchell C, et al. Genomewide association studies of suicide attempts in US soldiers. Am J Med Genet B Neuropsychiatr Genet. 2017; 174: 786–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Culpepper L. The diagnosis and treatment of bipolar disorder: decision-making in primary care. Prim Care Companion CNS Disord. 2014; 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Datto C, Pottorf WJ, Feeley L, LaPorte S, Liss C. Bipolar II compared with bipolar I disorder: baseline characteristics and treatment response to quetiapine in a pooled analysis of five placebo-controlled clinical trials of acute bipolar depression. Ann Gen Psychiatry. 2016; 15: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Charney AW, Ruderfer DM, Stahl EA, et al. Evidence for genetic heterogeneity between clinical subtypes of bipolar disorder. Transl Psychiatry. 2017; 7: e993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Markota M, Coombes BJ, Larrabee BR, et al. Association of schizophrenia polygenic risk score with manic and depressive psychosis in bipolar disorder. Transl Psychiatry. 2018; 8: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Musliner KL, Mortensen PB, McGrath JJ, et al. Association of polygenic liabilities for major depression, bipolar disorder, and schizophrenia with risk for depression in the Danish population. JAMA Psychiatry. 2019; 76: 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 2012; 9: 490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu G, Strathearn L, Liu B, Yang B, Bao W. Twenty-year trends in diagnosed attention-deficit/hyperactivity disorder among US children and adolescents, 1997–2016. JAMA Netw Open. 2018; 1: e181471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Faraone SV, Larsson H. Genetics of attention deficit hyperactivity disorder. Mol Psychiatry. 2019; 24: 562–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arnett AB, Pennington BF, Willcutt EG, DeFries JC, Olson RK. Sex differences in ADHD symptom severity. J Child Psychol Psychiatry. 2015; 56: 632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davies W. Sex differences in attention deficit hyperactivity disorder: candidate genetic and endocrine mechanisms. Front Neuroendocrinol. 2014; 35: 331–346. [DOI] [PubMed] [Google Scholar]
- 78.Martin J, Walters RK, Demontis D, et al. A genetic investigation of sex bias in the prevalence of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2018; 83: 1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ramtekkar UP, Reiersen AM, Todorov AA, Todd RD. Sex and age differences in attention-deficit/hyperactivity disorder symptoms and diagnoses: implications for DSM-V and ICD-11. J Am Acad Child Adolesc Psychiatry. 2010; 49: 217–228.e1-3. [PMC free article] [PubMed] [Google Scholar]
- 80.Polderman TJ, Hoekstra RA, Posthuma D, Larsson H. The co-occurrence of autistic and ADHD dimensions in adults: an etiological study in 17,770 twins. Transl Psychiatry. 2014; 4: e435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Skogli EW, Teicher MH, Andersen PN, Hovik KT, Oie M. ADHD in girls and boys–gender differences in co-existing symptoms and executive function measures. BMC Psychiatry. 2013; 13: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martin J, Taylor MJ, Rydell M, et al. Sex-specific manifestation of genetic risk for attention deficit hyperactivity disorder in the general population. J Child Psychol Psychiatry. 2018; 59: 908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Taylor MJ, Lichtenstein P, Larsson H, Anckarsater H, Greven CU, Ronald A. Is there a female protective effect against attention-deficit/hyperactivity disorder? Evidence from two representative twin samples. J Am Acad Child Adolesc Psychiatry. 2016; 55: 504–512.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Du Rietz E, Coleman J, Glanville K, Choi SW, O’Reilly PF, Kuntsi J. Association of polygenic risk for attention-deficit/hyperactivity disorder with co-occurring traits and disorders. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018; 3: 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brikell I, Larsson H, Lu Y, et al. The contribution of common genetic risk variants for ADHD to a general factor of childhood psychopathology. Mol Psychiatry 2018. [DOI] [PMC free article] [PubMed]
- 86.Ward J, Strawbridge RJ, Bailey MES, et al. Genome-wide analysis in UK biobank identifies four loci associated with mood instability and genetic correlation with major depressive disorder, anxiety disorder and schizophrenia. Transl Psychiatry. 2017; 7: 1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rabinowitz JA, Musci RJ, Milam AJ, et al. The interplay between externalizing disorders polygenic risk scores and contextual factors on the development of marijuana use disorders. Drug Alcohol Depend. 2018; 191: 365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wendt FR, Muniz Carvalho C, Pathak GA, Gelernter J, Polimanti R. . Deciphering the biological mechanisms underlying the genome-wide associations between computerized device use and psychiatric disorders. J Clin Med. 2019; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Olbert CM, Gala GJ, Tupler LA. Quantifying heterogeneity attributable to polythetic diagnostic criteria: theoretical framework and empirical application. J Abnorm Psychol. 2014; 123: 452–462. [DOI] [PubMed] [Google Scholar]
- 90.Zhou K, Chen W, Buitelaar J, et al. Genetic heterogeneity in ADHD: DAT1 gene only affects probands without CD. Am J Med Genet B Neuropsychiatr Genet. 2008; 147B: 1481–1487. [DOI] [PubMed] [Google Scholar]
- 91.Hu VW, Addington A, Hyman A. Novel autism subtype-dependent genetic variants are revealed by quantitative trait and subphenotype association analyses of published GWAS data. PLoS One. 2011; 6: e19067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hu VW, Steinberg ME. Novel clustering of items from the autism diagnostic interview-revised to define phenotypes within autism spectrum disorders. Autism Res. 2009; 2: 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu XQ, Paterson AD, Szatmari P. Genome-wide linkage analyses of quantitative and categorical autism subphenotypes. Biol Psychiatry. 2008; 64: 561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mandell DS, Novak MM, Zubritsky CD. Factors associated with age of diagnosis among children with autism spectrum disorders. Pediatrics. 2005; 116: 1480–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Masi A, DeMayo MM, Glozier N, Guastella AJ. An overview of autism spectrum disorder, heterogeneity and treatment options. Neurosci Bull. 2017; 33: 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chaste P, Klei L, Sanders SJ, et al. A genome-wide association study of autism using the Simons Simplex Collection: does reducing phenotypic heterogeneity in autism increase genetic homogeneity? Biol Psychiatry. 2015; 77: 775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gollwitzer A, Bargh JA. Social psychological skill and its correlates. Social Psychology. 2018; 49: 88–102. [Google Scholar]
- 98.Gollwitzer A, Martel C, McPartland JC, Bargh JA. Autism spectrum traits predict higher social psychological skill. Proc Natl Acad Sci U S A 2019; 16: 19245–19247. [DOI] [PMC free article] [PubMed]
- 99.Faridi F, Khosrowabadi R. Behavioral, cognitive and neural markers of asperger syndrome. Basic Clin Neurosci. 2017; 8: 349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kern JK, Geier DA, Sykes LK, Geier MR, Deth RC. Are ASD and ADHD a continuum? A comparison of pathophysiological similarities between the disorders. J Atten Disord. 2015; 19: 805–827. [DOI] [PubMed] [Google Scholar]
- 101.Robinson EB, Lichtenstein P, Anckarsater H, Happe F, Ronald A. Examining and interpreting the female protective effect against autistic behavior. Proc Natl Acad Sci U S A. 2013; 110: 5258–5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Baron-Cohen S. The extreme male brain theory of autism. Trends Cogn Sci. 2002; 6: 248–254. [DOI] [PubMed] [Google Scholar]
- 103.Geier DA, Kern JK, King PG, Sykes LK, Geier MR. An evaluation of the role and treatment of elevated male hormones in autism spectrum disorders. Acta Neurobiol Exp (Wars). 2012; 72: 1–17. [DOI] [PubMed] [Google Scholar]
- 104.Baron-Cohen S. Autism: the empathizing-systemizing (E-S) theory. Ann N Y Acad Sci. 2009; 1156: 68–80. [DOI] [PubMed] [Google Scholar]
- 105.Baron-Cohen S, Golan O, Ashwin E. Can emotion recognition be taught to children with autism spectrum conditions? Philos Trans R Soc Lond B Biol Sci. 2009; 364: 3567–3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wendt FR, Muniz Carvalho C, Gelernter J, Polimanti R. The effect of the genetic liability to autism spectrum disorder on emotion recognition in young unaffected probands from a population-based cohort. medRxiv. 2019; 19001230. [Google Scholar]
- 107.Takahashi N, Harada T, Nishimura T, et al. Association of genetic risks with autism spectrum disorder and early neurodevelopmental delays among children without intellectual disability. JAMA Netw Open. 2020; 3: e1921644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hannon E, Schendel D, Ladd-Acosta C, et al. Elevated polygenic burden for autism is associated with differential DNA methylation at birth. Genome Med. 2018; 10: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ranlund S, Rosa MJ, de Jong S, et al. Associations between polygenic risk scores for four psychiatric illnesses and brain structure using multivariate pattern recognition. Neuroimage Clin. 2018; 20: 1026–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jansen AG, Dieleman GC, Jansen PR, Verhulst FC, Posthuma D, Polderman TJC. Psychiatric polygenic risk scores as predictor for attention deficit/hyperactivity disorder and autism spectrum disorder in a clinical child and adolescent sample. Behav Genet 2019. [DOI] [PMC free article] [PubMed]
- 111.Goldstein RB, Smith SM, Chou SP, et al. The epidemiology of DSM-5 posttraumatic stress disorder in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. Soc Psychiatry Psychiatr Epidemiol. 2016; 51: 1137–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tolin DF, Foa EB. Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol Bull. 2006; 132: 959–992. [DOI] [PubMed] [Google Scholar]
- 113.Kessler RC, Aguilar-Gaxiola S, Alonso J, et al. Trauma and PTSD in the WHO World Mental Health Surveys. Eur J Psychotraumatol. 2017; 8: 1353383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Duncan LE, Ratanatharathorn A, Aiello AE, et al. Largest GWAS of PTSD (N 070) yields genetic overlap with schizophrenia and sex differences in heritability. Mol Psychiatry. 2018; 23: 666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Galatzer-Levy IR, Bryant RA. 636,120 Ways to have posttraumatic stress disorder. Perspect Psychol Sci. 2013; 8: 651–662. [DOI] [PubMed] [Google Scholar]
- 116.Kimerling R, Allen MC, Duncan LE. Chromosomes to social contexts: sex and gender differences in PTSD. Curr Psychiatry Rep. 2018; 20: 114. [DOI] [PubMed] [Google Scholar]
- 117.Lowe SR, Ratanatharathorn A, Lai BS, et al. Posttraumatic stress disorder symptom trajectories within the first year following emergency department admissions: pooled results from the International Consortium to predict PTSD. Psychol Med. 2020: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hasin DS, Sarvet AL, Meyers JL, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 2018; 75: 336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000; 157: 1552–1562. [DOI] [PubMed] [Google Scholar]
- 120.Martin J, Streit F, Treutlein J, et al. Expert and self-assessment of lifetime symptoms and diagnosis of major depressive disorder in large-scale genetic studies in the general population: comparison of a clinical interview and a self-administered checklist. Psychiatr Genet. 2017; 27: 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Howard DM, Adams MJ, Shirali M, et al. Genome-wide association study of depression phenotypes in UK biobank identifies variants in excitatory synaptic pathways. Nat Commun. 2018; 9: 1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hyde CL, Nagle MW, Tian C, et al. Identification of 15 genetic loci associated with risk of major depression in individuals of European descent. Nat Genet. 2016; 48: 1031–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ripke S, Wray NR, Lewis CM, et al. A mega-analysis of genome-wide association studies for major depressive disorder. Mol Psychiatry. 2013; 18: 497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Trzaskowski M, Mehta D, Peyrot WJ, et al. Quantifying between-cohort and between-sex genetic heterogeneity in major depressive disorder. Am J Med Genet B Neuropsychiatr Genet. 2019; 180: 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cardno AG, Owen MJ. Genetic relationships between schizophrenia, bipolar disorder, and schizoaffective disorder. Schizophr Bull. 2014; 40: 504–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Eaton WW, Martins SS, Nestadt G, Bienvenu OJ, Clarke D, Alexandre P. The burden of mental disorders. Epidemiol Rev. 2008; 30: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Khramtsova EA, Heldman R, Derks EM, Yu D, Davis LK, Stranger BE. Sex differences in the genetic architecture of obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2019; 180: 351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Browne HA, Gair SL, Scharf JM, Grice DE. Genetics of obsessive-compulsive disorder and related disorders. Psychiatr Clin North Am. 2014; 37: 319–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.International Obsessive Compulsive Disorder Foundation Genetics Collaborative (IOCDF-GC) and OCD Collaborative Genetics Association Studies (OCGAS). Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Mol Psychiatry. 2018; 23: 1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mattina GF, Steiner M. The need for inclusion of sex and age of onset variables in genetic association studies of obsessive-compulsive disorder: overview. Prog Neuropsychopharmacol Biol Psychiatry. 2016; 67: 107–116. [DOI] [PubMed] [Google Scholar]
- 131.Davis LK, Yu D, Keenan CL, et al. Partitioning the heritability of Tourette syndrome and obsessive compulsive disorder reveals differences in genetic architecture. PLoS Genet. 2013; 9: e1003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Huang MH, Cheng CM, Tsai SJ, et al. Familial coaggregation of major psychiatric disorders among first-degree relatives of patients with obsessive-compulsive disorder: a nationwide study. Psychol Med 2020: 1–8. [DOI] [PubMed]
- 133.Costas J, Carrera N, Alonso P, et al. Exon-focused genome-wide association study of obsessive-compulsive disorder and shared polygenic risk with schizophrenia. Transl Psychiatry. 2016; 6: e768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Neurodevelopmental Disorders. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. May 2013.
- 135.Robertson MM. The prevalence and epidemiology of Gilles de la Tourette syndrome. Part 1: the epidemiological and prevalence studies. J Psychosom Res. 2008; 65: 461–472. [DOI] [PubMed] [Google Scholar]
- 136.Khalifa N, von Knorring AL. Prevalence of tic disorders and Tourette syndrome in a Swedish school population. Dev Med Child Neurol. 2003; 45: 315–319. [DOI] [PubMed] [Google Scholar]
- 137.Scahill L, Specht M, Page C. The prevalence of tic disorders and clinical characteristics in children. J Obsessive Compuls Relat Disord. 2014; 3: 394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lichter DG, Finnegan SG. Influence of gender on Tourette syndrome beyond adolescence. Eur Psychiatry. 2015; 30: 334–340. [DOI] [PubMed] [Google Scholar]
- 139.Robertson MM. Movement disorders: Tourette syndrome–beyond swearing and sex? Nat Rev Neurol. 2014; 10: 6–8. [DOI] [PubMed] [Google Scholar]
- 140.Robertson MM. A personal 35 year perspective on Gilles de la Tourette syndrome: prevalence, phenomenology, comorbidities, and coexistent psychopathologies. Lancet Psychiatry. 2015; 2: 68–87. [DOI] [PubMed] [Google Scholar]
- 141.Jeon S, Walkup JT, Woods DW, et al. Detecting a clinically meaningful change in tic severity in Tourette syndrome: a comparison of three methods. Contemp Clin Trials. 2013; 36: 414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yang Z, Wu H, Lee PH, et al. Cross-disorder GWAS meta-analysis for attention deficit/hyperactivity disorder, autism spectrum disorder, obsessive compulsive disorder, and Tourette syndrome . bioRxiv. 2019; 770222. [Google Scholar]
- 143.Darrow SM, Hirschtritt ME, Davis LK, et al. Identification of two heritable cross-disorder endophenotypes for Tourette syndrome. Am J Psychiatry. 2017; 174: 387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kessler RC, Angermeyer M, Anthony JC, et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatry. 2007; 6: 168–176. [PMC free article] [PubMed] [Google Scholar]
- 145.Somers JM, Goldner EM, Waraich P, Hsu L. Prevalence and incidence studies of anxiety disorders: a systematic review of the literature. Can J Psychiatry. 2006; 51: 100–113. [DOI] [PubMed] [Google Scholar]
- 146.Davies MN, Verdi S, Burri A, et al. Generalised anxiety disorder – a twin study of genetic architecture, genome-wide association and differential gene expression. PLoS One. 2015; 10: e0134865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res. 2011; 45: 1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Aldao A, Mennin DS, Linardatos E, Fresco DM. Differential patterns of physical symptoms and subjective processes in generalized anxiety disorder and unipolar depression. J Anxiety Disord. 2010; 24: 250–259. [DOI] [PubMed] [Google Scholar]
- 149.Culpepper L. Generalized anxiety disorder and medical illness. J Clin Psychiatry. 2009; 70(Suppl 2): 20–24. [DOI] [PubMed] [Google Scholar]
- 150.Mussell M, Kroenke K, Spitzer RL, Williams JB, Herzog W, Lowe B. Gastrointestinal symptoms in primary care: prevalence and association with depression and anxiety. J Psychosom Res. 2008; 64: 605–612. [DOI] [PubMed] [Google Scholar]
- 151.Otowa T, Hek K, Lee M, et al. Meta-analysis of genome-wide association studies of anxiety disorders. Mol Psychiatry. 2016; 21: 1391–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Purves KL, Coleman JRI, Meier SM, et al. A major role for common genetic variation in anxiety disorders. Mol Psychiatry 2019. [DOI] [PMC free article] [PubMed]
- 153.Flint AJ, Peasley-Miklus C, Papademetriou E, et al. Effect of age on the frequency of anxiety disorders in major depression with psychotic features. Am J Geriatr Psychiatry. 2010; 18: 404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lenze EJ, Wetherell JL. A lifespan view of anxiety disorders. Dialogues Clin Neurosci. 2011; 13: 381–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bruce LC, Heimberg RG, Blanco C, Schneier FR, Liebowitz MR. Childhood maltreatment and social anxiety disorder: implications for symptom severity and response to pharmacotherapy. Depress Anxiety. 2012; 29: 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Iffland B, Sansen LM, Catani C, Neuner F. Emotional but not physical maltreatment is independently related to psychopathology in subjects with various degrees of social anxiety: a web-based internet survey. BMC Psychiatry. 2012; 12: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Stein MB, Schork NJ, Gelernter J. Gene-by-environment (serotonin transporter and childhood maltreatment) interaction for anxiety sensitivity, an intermediate phenotype for anxiety disorders. Neuropsychopharmacology. 2008; 33: 312–319. [DOI] [PubMed] [Google Scholar]
- 158.Centers for Disease Control and Prevention. Web-Based Injury Statistics Query and Reporting System (WISQARS). Atlanta, GA: Author; 2013.
- 159.Centers for Disease Control and Prevention. Suicide Facts at a Glance. Atlanta, GA: Author; 2015.
- 160.Bradvik L. Suicide risk and mental disorders. Int J Environ Res Public Health. 2018; 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nordentoft M, Mortensen PB, Pedersen CB. Absolute risk of suicide after first hospital contact in mental disorder. Arch Gen Psychiatry. 2011; 68: 1058–1064. [DOI] [PubMed] [Google Scholar]
- 162.Mann JJ, Waternaux C, Haas GL, Malone KM. Toward a clinical model of suicidal behavior in psychiatric patients. Am J Psychiatry. 1999; 156: 181–189. [DOI] [PubMed] [Google Scholar]
- 163.Zai CC, de Luca V, Strauss J, Tong RP, Sakinofsky I, Kennedy JL. Genetic factors and suicidal behavior In: Dwivedi Y, ed. The Neurobiological Basis of Suicide. Boca Raton, FL: CRC Press/Taylor & Francis; 2012. [PubMed] [Google Scholar]
- 164.Mullins N, Bigdeli TB, Borglum AD, et al. GWAS of suicide attempt in psychiatric disorders and association with major depression polygenic risk scores. Am J Psychiatry. 2019; 176: 651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Otsuka I, Akiyama M, Shirakawa O, et al. Genome-wide association studies identify polygenic effects for completed suicide in the Japanese population. Neuropsychopharmacology. 2019; 44: 2119–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Strawbridge RJ, Ward J, Ferguson A, et al. Identification of novel genome-wide associations for suicidality in UK biobank, genetic correlation with psychiatric disorders and polygenic association with completed suicide. EBioMedicine. 2019; 41: 517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Levey DF, Polimanti R, Cheng Z, et al. Genetic associations with suicide attempt severity and genetic overlap with major depression. Transl Psychiatry. 2019; 9: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Polimanti R, Levey D, Pathak G, et al. Complex multi-environment gene interactions linked to the interplay between polysubstance dependence and suicidal behaviors. medRxiv 2020; 2020.01.14.20017509. [DOI] [PMC free article] [PubMed]
- 169.Udo T, Grilo CM. Prevalence and correlates of DSM-5-defined eating disorders in a nationally representative sample of U.S. adults. Biol Psychiatry. 2018; 84: 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Spindler A, Milos G. Links between eating disorder symptom severity and psychiatric comorbidity. Eat Behav. 2007; 8: 364–373. [DOI] [PubMed] [Google Scholar]
- 171.Breithaupt L, Hubel C, Bulik CM. Updates on genome-wide association findings in eating disorders and future application to precision medicine. Curr Neuropharmacol. 2018; 16: 1102–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Duncan L, Yilmaz Z, Gaspar H, et al. Significant locus and metabolic genetic correlations revealed in genome-wide association study of anorexia nervosa. Am J Psychiatry. 2017; 174: 850–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wong CCY, Schumann G. Review. Genetics of addictions: strategies for addressing heterogeneity and polygenicity of substance use disorders. Philos Trans R Soc Lond B Biol Sci. 2008; 363: 3213–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Polimanti R, Walters RK, Johnson EC, et al. Leveraging genome-wide data to investigate differences between opioid use vs. opioid dependence in 41,176 individuals from the Psychiatric Genomics Consortium. Mol Psychiatry 2020. [DOI] [PMC free article] [PubMed]
- 175.Substance Abuse and Mental Health Services Administration. CBHSQ Methodology Report. Impact of the DSM-IV to DSM-5 Changes on the National Survey on Drug Use and Health. Rockville, MD: Author; 2016. [PubMed] [Google Scholar]
- 176.Hunt GE, Malhi GS, Cleary M, Lai HM, Sitharthan T. Comorbidity of bipolar and substance use disorders in national surveys of general populations, 1990-2015: systematic review and meta-analysis. J Affect Disord. 2016; 206: 321–330. [DOI] [PubMed] [Google Scholar]
- 177.Verhulst B, Neale MC, Kendler KS. The heritability of alcohol use disorders: a meta-analysis of twin and adoption studies. Psychol Med. 2015; 45: 1061–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kranzler HR, Zhou H, Kember RL, et al. Genome-wide association study of alcohol consumption and use disorder in 274,424 individuals from multiple populations. Nature Communications. 2019; 10: 1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hancock DB, Guo Y, Reginsson GW, et al. Genome-wide association study across European and African American ancestries identifies a SNP in DNMT3B contributing to nicotine dependence. Mol Psychiatry. 2018; 23: 1911–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Erzurumluoglu AM, Liu M, Jackson VE, et al. Meta-analysis of up to 622,409 individuals identifies 40 novel smoking behaviour associated genetic loci. Mol Psychiatry 2019. [DOI] [PMC free article] [PubMed]
- 181.Hancock DB, Markunas CA, Bierut LJ, Johnson EO. Human genetics of addiction: new insights and future directions. Curr Psychiatry Rep. 2018; 20: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pasman JA, Verweij KJH, Gerring Z, et al. GWAS of lifetime cannabis use reveals new risk loci, genetic overlap with psychiatric traits, and a causal effect of schizophrenia liability (vol 21, pg 1161, 2018). Nat Neurosci. 2019; 22: 1196. [DOI] [PubMed] [Google Scholar]
- 183.Minica CC, Verweij KJH, van der Most PJ, et al. Genome-wide association meta-analysis of age at first cannabis use. Addiction. 2018; 113: 2073–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sherva R, Wang Q, Kranzler H, et al. Genome-wide association study of cannabis dependence severity, novel risk variants, and shared genetic risks. JAMA Psychiatry. 2016; 73: 472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jons WA, Colby CL, McElroy SL, Frye MA, Biernacka JM, Winham SJ. Statistical methods for testing X chromosome variant associations: application to sex-specific characteristics of bipolar disorder. Biol Sex Differ. 2019; 10: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Luciano M, Davies G, Summers KM, et al. The influence of X chromosome variants on trait neuroticism. Mol Psychiatry 2019. [DOI] [PMC free article] [PubMed]
- 187.Vousooghi N, Shirazi MS, Goodarzi A, Abharian PH, Zarrindast MR. X chromosome inactivation in opioid addicted women. Basic Clin Neurosci. 2015; 6: 179–184. [PMC free article] [PubMed] [Google Scholar]
- 188.Espeso-Gil S, Halene T, Bendl J, et al. A chromosomal connectome for psychiatric and metabolic risk variants in adult dopaminergic neurons. Genome Med. 2020; 12: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Giusti-Rodríguez P, Lu L, Yang Y, et al. Using three-dimensional regulatory chromatin interactions from adult and fetal cortex to interpret genetic results for psychiatric disorders and cognitive traits. bioRxiv 2019; 406330.
- 190.Zhang X, Zhang Y, Zhu X, et al. Local and global chromatin interactions are altered by large genomic deletions associated with human brain development. Nat Commun. 2018; 9: 5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ford JM, Morris SE, Hoffman RE, et al. Studying hallucinations within the NIMH RDoC framework. Schizophr Bull. 2014; 40(Suppl 4): S295–S304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Peterson RE, Kuchenbaecker K, Walters RK, et al. Genome-wide association studies in ancestrally diverse populations: opportunities, methods, pitfalls, and recommendations. Cell. 2019; 179: 589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]