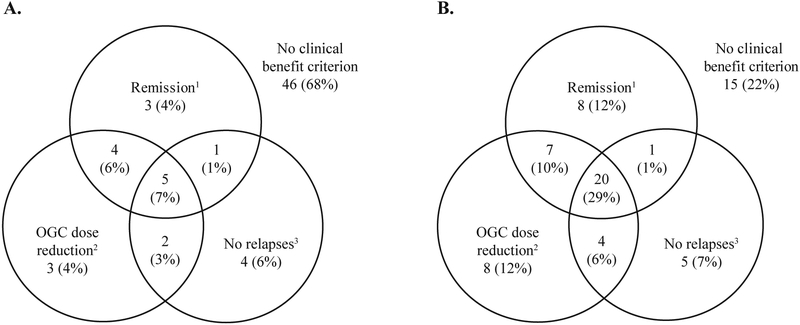
Figure 2.
Summary of the proportion of patients receiving A. placebo (N=68) and B. mepolizumab (N=68) to meet each definition of clinical benefit.
1Remission category BVAS=0 and OGC dose ≤4 mg/day during the study treatment period; 2≥50% reduction in average OGC dose during Weeks 48–52; 3No EGPA relapses during the study treatment period.
BVAS; Birmingham Vasculitis Activity Score; OGC, oral glucocorticoid.