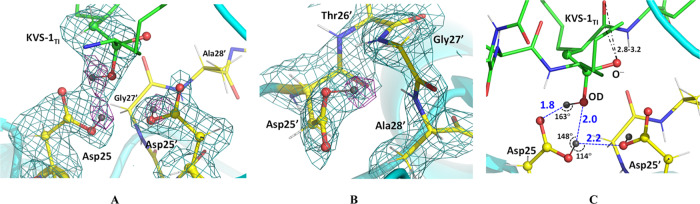
Figure 4.
(A) The catalytic site of the HIV-1 PR/KVS-1TI complex. (B) Catalytic Asp25′, and residues Thr26′, Gly27′, and Ala28′ making a small hydrophobic pocket where D bonded to the Asp25′ carboxylic group is facing. (C) Hydrogen bonds (blue dashed lines with O···D distances in Å, and O-D···O angles in deg.) made between the catalytic Asp dyad and the oxygen atoms of the intermediate. The negatively charged oxygen atom of the oxyanion makes close 2.8–3.2 Å contacts with next carbonyl in the hexapeptide main chain. For panels A and B, 2FO-FC neutron scattering length density map at a 2.2 Å resolution is contoured at the 1.5 σ level; the FO-FC-omit difference neutron scattering length density map is the violet mesh contoured at the 3 σ level, indicating the locations of the three D atoms (dark gray spheres) involved in catalysis (other D atoms are light gray). H atoms of KVS-1TI are omitted for clarity.