Figure 4.
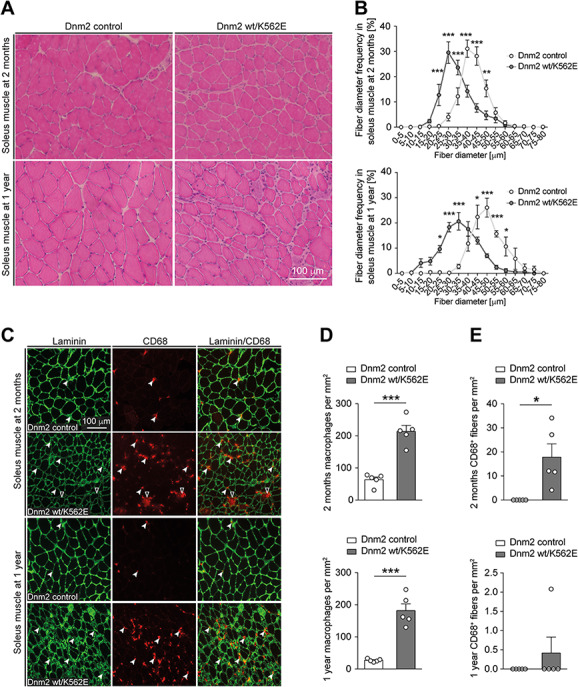
The Dnm2 wt/K562E mouse develops an evident myopathy. (A) Exemplary H&E-stained cryosections of soleus muscles derived from control and Dnm2 wt/K562E mice at 2 months and 1 year of age. Scale bar: 100 μm, refers to whole panel. (B) Quantification of myofibre diameter frequencies reveals a shift towards smaller-calibre fibres in Dnm2 wt/K562E soleus muscles at both 2 months and 1 year of age. Datapoints, mean; error bars, SEM (n = 5 mice/genotype, images from three sections quantified and averaged per mouse). (C) Exemplary soleus muscle cryosections from control and Dnm2 wt/K562E probed with antibodies targeting laminin or CD68 at both 2 months and 1 year of age. Exemplary CD68-positive macrophages in between myofibres (white arrowheads) and from within the myofibre basal lamina (black arrowheads with white lining) are highlighted. Scale bar: 100 μm, refers to whole panel. (D, E) Quantification of CD68-positive macrophages in between myofibres (D) reveals a significant enrichment in Dnm2 wt/K562E mice at both 2 months and 1 year, and quantification of CD68-positive macrophages from within myofibre basal lamina (E) reveals a significant increase in Dnm2 wt/K562E soleus muscles at 2 months. Bar heights, mean; error bars, SEM (n = 5 mice/genotype, images of at least three sections quantified and averaged per mouse). Two-way ANOVA with Sidak’s multiple comparisons test (B), two-tailed unpaired Student’s t-test (D) and one-sample t-test (E). Significance was set at *P < 0.05, **P < 0.01 and ***P < 0.001.
