Abstract
In a previous study, it was demonstrated that T-cell immune response cDNA 7 (TIRC7) levels reflect the efficacy of treatment of patients with acute graft-versus-host disease (GVHD). However, the pathogenesis of TIRC7 in acute GVHD remains poorly understood. Lymphocytes from patients with acute GVHD were selected as targeT cells, and the effects of TIRC7 on cytotoxic T lymphocyte antigen-4 (CTLA-4), T cell activation and cytokine secretion were observed by electroporation. A mouse model of acute GVHD was established; anti-TIRC7 and anti-CTLA-4 monoclonal antibodies were intraperitoneally injected into recipient mice. Then, the effects of TIRC7 and CTLA-4 on T cell activation and acute GVHD were monitored. After TIRC7 expression was downregulated, CTLA-4 levels were decreased and STAT3 phosphorylation was reduced; conversely, the activation capacity of T lymphocytes was elevated, and the secretion of interferon-γ and other cytokines was increased. The mice in the TIRC7 + CTLA-4 co-administration group exhibited the lowest acute GVHD scores, with the longest average survival time and shortest recovery time of hematopoietic reconstitution. In conclusion, the results indicated that TIRC7 may positively regulate the function of CTLA-4 and inhibit T cell activation, thus suppressing the development and progression of acute GVHD.
Keywords: T-cell immune response cDNA 7, T helper 1 cells, cytotoxic T lymphocyte antigen-4, STAT3, acute graft-versus-host disease
Introduction
Acute graft-versus-host disease (GVHD) is a serious complication following allogeneic hematopoietic stem cell transplantation (1); at present, its pathogenesis remains unclear. A number of studies have demonstrated that T lymphocyte activation is the initial factor for the occurrence of acute GVHD (2–4). Therefore, inhibition of T cell activation may ameliorate acute GVHD.
Cytotoxic T lymphocyte antigen-4 (CTLA-4) is a transmembrane protein expressed on T cells and is a major inhibitory receptor on T cells (5). It transfers inhibitory signals to activated T cells to reduce their level of activation (5). A further study revealed that during acute GVHD, expression of CTLA-4 is downregulated, leading to enhanced T cell activation (6). Yoo et al (7) demonstrated that in a mouse model of acute GVHD, following overexpression of CTLA-4 in T cells, the degree of T cell activation declined and the apoptosis of T cells increased, resulting in a decreased severity of acute GVHD. These studies indicated that CTLA-4 may play a negative role in the regulation of acute GVHD.
It has previously been reported that the expression of T-cell immune response cDNA 7 (TIRC7) is increased in patients with acute GVHD and decreased following treatment, and that with the progression of acute GVHD, there are higher expression levels of inducible TIRC7 (8); previous studies have reported that TIRC7 is the upstream regulatory molecule of CTLA-4 (9–11). However, whether TIRC7 modulates the development and progression of acute GVHD by regulating CTLA-4 remains poorly understood.
The present study demonstrated that when TIRC7 expression was downregulated, CTLA-4 levels were decreased and STAT3 phosphorylation was reduced, with elevated activation of T lymphocytes, and secretion of interferon (IFN)-γ and other cytokines. In the in vivo experiment, the mice injected with antibodies against TIRC7 and CTLA-4 had the lowest acute GVHD scores, longest average survival time and shortest hematopoietic reconstitution recovery time. These findings suggested that TIRC7 decreases the development and progression of acute GVHD by regulating CTLA-4 and T cell activation.
Materials and methods
Separation and activation of CD4+ T lymphocytes
Peripheral blood mononuclear cells were isolated from patients with acute GVHD using Ficoll-Paque Plus (Sinopharm Chemical Reagent Co., Ltd.). For each experiment, 1×107 cells/ml were resuspended in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal calf serum (Gibco; Thermo Fisher Scientific, Inc.). CD4+ T lymphocytes were purified with negative selection using magnetic beads according to the manufacturer's protocol (Miltenyi Biotec, Inc.), and then CD4+ T cells were generated by stimulation with anti-CD3 and anti-CD28 Dynabeads (Invitrogen; Thermo Fisher Scientific, Inc.) for 3–7 days. Written informed consent was provided by all participants included in the present study. Ethical approval for the present study was obtained from the Medical Ethics Committee of the Affiliated Hospital of Xuzhou Medical University.
Construction of pGPU6-shTIRC7 and FLAG-CTLA-4
The present study obtained the cDNA sequence of the TIRC7 gene from GeneBank (NM_006019.3) and designed two short hairpin (sh)RNAs for TIRC7 and one non-specific sequence (control group) using Primer 5.0 (Premier Biosoft International). After the oligonucleotide fragments were synthesized by Invitrogen (Thermo Fisher Scientific, Inc.), these fragments were inserted into a pGPU6/Neo linearized vector digested by EcoRI and KpnI. The plasmids pGPU6-shTIRC7 and pGPU6-shcontrol were constructed and sequenced by Invitrogen (Thermo Fisher Scientific, Inc.) following the transformation of competenT cells. The primers of shTIRC7-1, shTIRC7-2 and shcontrol were as follows: shTIRC7-1, 5′-CACCGGACCTGAGGGTCAACTTTGTTTCAAGAGAACAAAGTTGACCCTCAGGTCCTTTTTTG-3′ and 5′-GATCCAAAAAGGACCTGAGGGTCAACTTTGTTCTCTTGAAACAAAGTTGACCCTCAGGTCC-3′; shTIRC7-2, 5′-CACCGCTTCCTCATTGCCAGCTTCATTCAAGAGATGAAGCTGGCAATGAGGAAGCTTTTTTG-3′ and 5′-GATCCAAAAAAGCTTCCTCATTGCCAGCTTCATCTCTTGAATGAAGCTGGCAATGAGGAAGC-3′; shcontrol, 5′-CACCGTTCTCCGAACGTGTCACGTCAAGAGATTACGTGACACGTTCGGAGAATTTTTTG-3′ and 5′-GATCCAAAAAATTCTCCGAACGTGTCACGTAATCTCTTGACGTGACACGTTCGGAGAAC-3′. cDNA was obtained from peripheral blood mononuclear cells of patients with acute GVHD, and the full-length sequence of human CTLA-4 cDNA was cloned into a CMV expression vector p3×FLAG-CMV™-14 and termed FLAG-CTLA-4. CTLA-4 primers were: Forward, 5′-GGGAATTCATGGCTTGCCTTGGATTTC-3′ and reverse, 5′-GGGGTACCCGATTGATGGGAATAAAATAAGG-3′. The sequence of FLAG-CTLA-4 was validated by Invitrogen (Thermo Fisher Scientific, Inc.). PCR was performed using a polymerase purchased from Invitrogen (cat. no. F531S; Thermo Fisher Scientific, Inc.). The following conditions for PCR were set: 94°C for 2 min, followed by 35 cycles of 94°C for 30 sec, 61°C for 30 sec and 72°C for 2 min, with a final extension at 72°C for 10 min.
CD4+ T cells transfected by electroporation
The activated CD4+ T cells (2×107 cells) were transfected transiently with pGPU6-shTIRC7/FLAG-CTLA-4 (10 µg/well) by electroporation methods using a Neon™ device according to the manufacturer's protocol (Invitrogen; Thermo Fisher Scientific, Inc.). Cells were cultured in RPMI-1640 medium; expression of the plasmid was selected for using G418 (500 µg/ml; Gibco; Thermo Fisher Scientific, Inc.). After culture for 48 h, RNA and protein were collected and monitored by reverse transcription-quantitative PCR (RT-qPCR) and western blotting.
Measurement of TIRC7 and CTLA-4 via western blotting
The transfected CD4+ T cells were collected and lysed using cell lysis buffer (cat. no. 9803; Cell Signaling Technology, Inc.). Protein concentrations were determined via the bicinchoninic acid method, and protein (30 µg/lane) was separated via 5–10% SDS-PAGE. Nitrocellulose membranes were blocked using 5% bovine serum albumin (cat. no. V900933; Sigma-Aldrich; Merck KGaA) in TBS-0.05% Tween-20 for 1 h at room temperature, and then underwent western blotting analysis using anti-CTLA-4 monoclonal antibody (1 µg/ml; cat. no. ab110650; Abcam) with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (1:10,000; cat. no. AP124P; EMD Millipore) as the secondary antibody. TIRC7 protein level was measured by anti-TIRC7 polyclonal antibody (1 µg/ml; cat. no. sc-293491; Santa Cruz Biotechnology, Inc.) with HRP-conjugated goat anti-mouse IgG (1:5,000; cat. no. AP124P; EMD Millipore) as the secondary antibody. The temperature and duration of primary and secondary antibody incubations were overnight at 4°C and 2 h at room temperature, respectively. An ECL detection system was purchased from Thermo Fisher Scientific, Inc. (cat. no. 32106).
RT-qPCR to measure TIRC7 and CTLA-4
RT-qPCR analysis was performed as previously described (12). RNA was extracted from the peripheral blood of patients using Ficoll-Paque Plus (Sinopharm Chemical Reagent Co., Ltd.) and reverse transcribed to cDNA using a PrimeScript RT kit (Takara Bio, Inc.) according to the manufacturer's protocols. Then, qPCR was performed using LightCycler480 (Roche Diagnostics). The qPCR system (20 µl) was established in triplicate as follows: 10 µl SYBR® Green Supermix (Bio-Rad Laboratories, Inc.), 5 µl cDNA, 4 µl ddH2O, 0.5 µl forward primer (10 µmol/l) and 0.5 µl reverse primer (10 µmol/l). The following conditions were used: 94°C for 2 min, followed by 35 cycles of 94°C for 30 sec, 60°C for 30 sec and 72°C for 35 sec, and a final extension at 72°C for 2 min. The quantification cycle (Cq) was calculated using the 2−ΔΔCq method (13). The primers of TIRC7 were forward, 5′-TTTGCTGTGTTGACTGTGGC-3′ and reverse, 5′-CACTTCGGAGAAGCAGGGATT-3′. The primers of CTLA-4 were forward, 5′-TGTGCCACGACATTCACAGA-3′ and reverse, 5′-CATGCCCACAAAGTATGGCG-3′. Forward and reverse primers of β-actin were 5′-ATGGAGGGGAATACAGCCC-3′ and 5′-TTCTTTGCAGCTCCTTCGTT-3′, respectively.
Measurement of STAT3
The luciferase reporter gene pGL-3-STAT3-luciferase (GLSTAT3-Lu) was synthesized by Beijing Yuan Ping Hao Biotechnology Co., Ltd. pGL-3-Basic was used in the control group. After GLSTAT3-Lu/pGL-3-Basic (10 µg/well) and FLAG-CTLA-4/FLAG (10 µg/well) were transfected into CD4+ T cells (2×107 cells) by electroporation methods using a Neon™ device according to the manufacturer's protocol (Invitrogen; Thermo Fisher Scientific, Inc.) for 48 h, luciferase reporter gene activity was assessed using a dual-luciferase reporter gene assay kit, according to the manufacturer's protocol (Promega Corporation). The luciferase detection device used was a 96-microplate luminometer (Promega Corporation) and secreted alkaline phosphatase (cat. no. KA1362; Abnova) was used to normalize luciferase activity. Each experiment was repeated three times. STAT3 and phosphorylated (p)STAT3 protein levels were also monitored via western blotting. Anti-STAT3 (1:5,000; cat. no. ab119352) and anti-pSTAT3 (1:10,000; cat. no. ab76315) were both obtained from Abcam.
Proliferation of CD4+ T cells
Transfected CD4+ T cells were collected and the concentration was adjusted to 2×105 cells/ml. Cells were added to 96-well plates with 100 µl in each well and incubated at 37°C and 5% CO2. At 24, 48 and 72 h after incubation, Cell Counting Kit-8 (CCK-8; Takara Bio, Inc.) was added (10 µl/well). The optical density at 450 nm was measured using a microplate reader (Thermo Fisher Scientific, Inc.) at 4 h after adding CCK-8.
Flow cytometry
CD4+ T cells transfected with FLAG-CTLA-4 were collected and a total of 1×107 cells were suspended in 2 ml medium. Phycoerythrin (PE)-conjugated pSTAT antibody (1:50; cat. no. 612569; BD Biosciences) was incubated for 1 h at room temperature. The data were collected using FACSCalibur (BD Biosciences) and analyzed by CellQuest software version 5.1 (BD Biosciences).
Annexin V (eBioscience; Thermo Fisher Scientific, Inc.) and 7-aminoactinomycin D (7-AAD; eBioscience; Thermo Fisher Scientific, Inc.) were utilized to label apoptotic CD4+ T cells. Incubation with Annexin V was performed at room temperature for 15 min. Analysis was performed on FACSCalibur using CellQuest software version 5.1. Annexin V-positive and 7-AAD-negative cells were defined as apoptotic cells, and the cells without added Annexin V and 7-AAD were used as the negative control group.
Analysis of Th cells via flow cytometry was performed as previously described (14). Anti-CD3-FITC (cat. no. 11-0037-42), anti-CD8-peridinin-chlorophyll (PerCP)/cyanine 5.5 (cat. no. 45-0081-82), PE-conjugated anti-human interleukin (IL)-17 (cat. no. 12-7177-81) and IL-22 (cat. no. 12-7229-42), and allophycocyanin (APC)-conjugated anti-human IFN-γ (cat. no. 17-7319-82) and anti-human IL-4 (cat. no. 17-7041-82) were all purchased from eBioscience (all 1:20; Thermo Fisher Scientific, Inc.). Cells were fixed in 100 µl fixative solution (4% formaldehyde in PBS; cat. no. R37602; eBioscience; Thermo Fisher Scientific, Inc.) for 15 min at room temperature, before 100 µl permeabilization solution (0.5% Triton X-100; cat. no. R37602) was added for 5 min at room temperature. Phorbol myristate acetate (1 µl/ml), ionomycin (1 µl/ml) and brefeldin A (2 µl/ml) were obtained from Sigma Aldrich (Merck KGaA) and incubated with cells at 37°C for 4–6 h. Antibody incubations were performed at room temperature for 15 min. The analysis was performed using FACSCalibur and these data were analyzed by CellQuest software version 5.1.
Mice
Specific pathogen-free C57BL/6 mice (H-2Kb; age, 8–12 weeks; 18–22 g; 20 male mice) were selected as donor mice with BALB/c mice (H-2Kd; age, 8–12 weeks; 18–22 g; 172 male mice) as recipients. The mice were purchased from Shanghai SLAC Laboratory Animal Co. Ltd. Mice were housed in sterilized microisolator cages and maintained in the individually ventilated cage room of the Experimental Animal Center of Xuzhou Medical University. The temperature and relative humidity of the room were 19–21°C and ~50%, respectively. Animals were maintained on an 12:12-h light/dark cycle. Water was autoclaved, and feed was purchased from Shanghai Pluton Biotechnology Co., Ltd. Food and water were provided ad libitum. Then, they were kept on autoclaved acidified water (pH 2.5) for the week before transplantation and the first week after transplantation. The experiments were approved by the Animal Committee of Xuzhou Medical University and all protocols were performed in accordance with the Institutional Animal Care and Use Committee guidelines.
Acute GVHD mice model
C57BL/6 mice were sacrificed by cervical dislocation, immersed in iodine volts for 5 min, and the tibia and femur were aseptically separated. After removing the attached muscles and fascia, the metaphysis was cut open. The bone marrow cavity was washed with PBS. Then, a single cell suspension was produced by filtering through a 220-mesh stainless steel strainer. The bone marrow cells were prepared following centrifugation at 4°C and 800 × g for 5 min and suspended in PBS buffer. BALB/c mice were given 7.5 Gy lethal total body irradiation [total body irradiation (TBI), 60Co γ-ray source at 0.66 Gy/min] and injected with bone marrow cells isolated from C57BL/6 mice via the tail vein within 6 h. The mice were randomly divided into 10 groups as follows: i) Transplantation control (control) group, normal saline; ii) TBI group, no cell infusion; iii) A1 group, donor bone marrow cells (5×106/mouse) + splenic lymphocytes (5×105/mouse); iv) A2 group, donor bone marrow cells (5×106/mouse) + splenic lymphocytes (5×105/mouse) + CTLA-4 monoclonal antibody (40 µg/day); v) A3 group, donor bone marrow cells (5×106/mouse) + splenic lymphocytes (5×105/mouse) + TIRC7 monoclonal antibody (25 µg/day); vi) A4 group, donor bone marrow cells (5×106/mouse) + splenic lymphocytes (5×105/mouse) + CTLA-4 monoclonal antibody (40 µg/day) + TIRC7 monoclonal antibody (25 µg/day); vii) B1 group, donor bone marrow cells (5×106/mouse) + splenic lymphocytes (5×106/mouse); viii) B2 group, donor bone marrow cells (5×106/mouse) + splenic lymphocytes (5×106/mouse) + CTLA-4 monoclonal antibody (40 µg/day); ix) B3 group, donor bone marrow cells (5×106/mouse) + splenic lymphocytes (5×106/mouse) + TIRC7 monoclonal antibody (25 µg/day); and x) B4 group, donor bone marrow cells (5×106/mouse) + splenic lymphocytes (5×106/mouse) + CTLA-4 monoclonal antibody (40 µg/day) + TIRC7 monoclonal antibody (25 µg/day). There were 6 mice in the control and TBI groups, and 20 mice in each of the last 8 groups. CTLA-4 and TIRC7 monoclonal antibodies were custom-generated by Wuhan GeneCreate Biological Engineering Co., Ltd., and administered by intraperitoneal injection. The optimal dosing time of CTLA-4 antibody was day 0 post-transplantation and its optimal dose was 40 µg/mouse; the optimal dose and dosing time of TIRC7 antibody was 25 µg/mouse and day 0, 1, 2, 3, 4 and 7 post-transplantation.
Observation index post-transplantation
The survival of mice was monitored daily and their survival times were recorded. The degree of clinical acute GVHD was assessed weekly using a scoring system (15) that integrates the following clinical parameters: Weight loss, posture, activity, fur and skin integrity. A total of 30 days after the transplantation, FITC-anti-H-2Kd (0.1 mg/ml; cat. no. MA5-18010) and PE-anti-H-2Kb (0.1 mg/ml; cat. no. MA5-18000) monoclonal antibodies (eBioscience; Thermo Fisher Scientific, Inc.) were used to detect the allogenic chimerism of transplanted mice by flow cytometry. On days 7, 14, 21, 28 and 35 post-transplantation, 3 mice/group were sacrificed by cervical dislocation; liver, lung and colon tissues obtained from the mice in each group were examined. Mice with pathological changes indicative of acute GVHD were considered as acute GVHD, and changes were scored according to the Blazar and Kaplan acute GVHD pathological scoring system (16,17) for the liver, lung and colon. Blood of the recipients in each group was collected every 7 days post-transplantation. Cells were stained with anti-mouse IFN-γ-APC and anti-mouse IL-17a-PE, or anti-mouse IL-4-APC and anti-mouse IL-22-PE. Plasma was isolated from blood samples prepared following centrifugation at 4°C and 800 × g for 5 min. The levels of T helper (Th) cells were monitored by flow cytometry. TIRC7 (cat. no. 12649-1-AP; ProteinTech Group, Inc.) and CTLA-4 (cat. no. KA3352; Abnova) plasma levels were monitored by ELISA; the relative levels of TIRC7 and CTLA-4 in mononuclear cells from blood samples were monitored by qPCR.
Statistical analysis
All data were statistically analyzed using SPSS 19.0 software (IBM Corp.). The mean ± standard deviation, as well as the range and median, were calculated for each variable from three experimental repeats. Kruskal-Wallis test and Dunn's test were performed to compare factors, such as white blood cell counts and GVHD scores in different groups. All P-values were two-tailed. P<0.05 was considered to indicate a statistically significant difference.
Results
Regulation of the expression of CTLA-4 by TIRC7
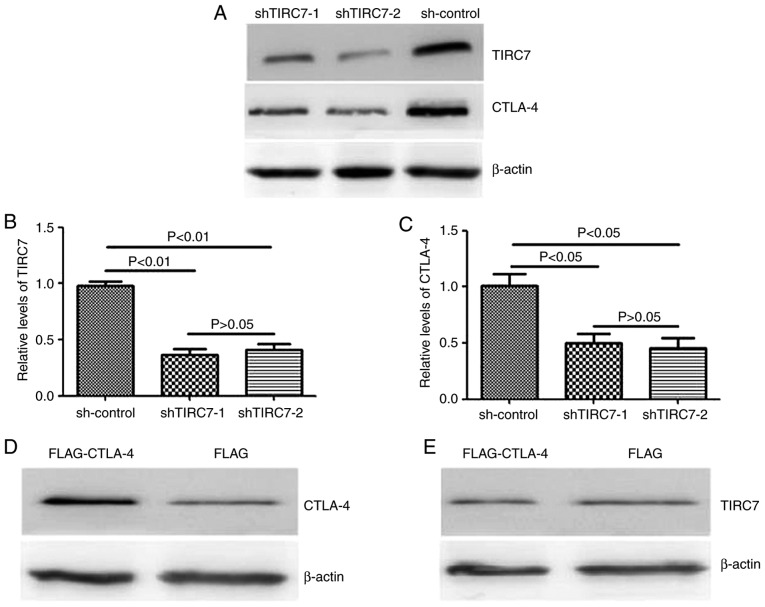
pGPU6-shTIRC7 was transfected into CD4+ T cell by electroporation in order to analyze the regulatory effects of TIRC7 on CTLA-4 expression using western blotting and qPCR. It was revealed that in the shTIRC7-1 and shTIRC7-2 groups, TIRC7 and CTLA-4 protein levels were significantly reduced compared with the sh-control group (P<0.05); however, there was no significant difference between the shTIRC7-1 and shTIRC7-2 groups (P>0.05; Fig. 1A-C).
Figure 1.
Reciprocal effects of TIRC7 and CTLA-4 expression on the expression of CTLA-4 and TIRC7. (A) TIRC7 and CTLA-4 protein expression levels were analyzed via western blotting after shRNA transfection. Expression of (B) TIRC7 and (C) CTLA-4 were evaluated after shRNA transfection via quantitative PCR. Expression of (D) CTLA-4 and (E) TIRC7 in FLAG-CTLA-4 and FLAG-transfected cells was monitored via western blotting. CLTA-4, cytotoxic T lymphocyte antigen-4; TIRC7, T-cell immune response cDNA 7; sh(RNA), short hairpin (RNA).
In addition, CD4+ T cells from the peripheral blood of patients with acute GVHD were transfected with FLAG- CTLA-4 to ascertain whether the regulation of CTLA-4 was affected by the expression of TIRC7. The present study demonstrated that the expression of CTLA-4 in the FLAG-CTLA-4 group was markedly higher than that in the control vector-infected group; however, compared with the FLAG group, there was no notable difference in the TIRC7 expression level in the FLAG-CTLA-4 group (Fig. 1D and E), suggesting that CTLA-4 had no effect on the expression of TIRC7.
Effects of TIRC7 knockdown on STAT3 luciferase activity, and STAT3 protein expression and phosphorylation
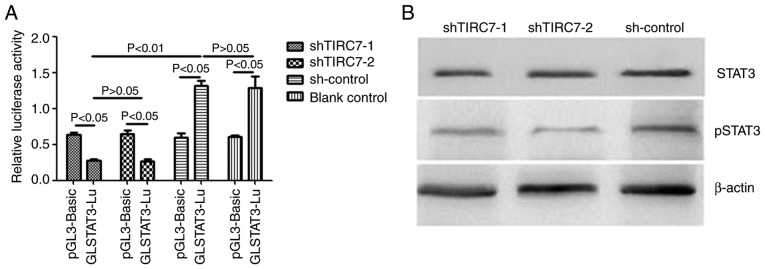
The luciferase reporter plasmid GLSTAT3-Lu/pGL-3-basic and shRNA plasmids were co-transfected into the CD4+ T cells of patients with acute GVHD via electroporation. After 48 h of culture, the cells were collected and analyzed using a dual-luciferase reporter gene system. As presented in Fig. 2A, the luciferase activity of GLSTAT3-Lu in the shTIRC7-1 and shTIRC7-2 groups were both lower than that of pGL-3-Basic in the shTIRC7-1 and shTIRC7-2 groups (P<0.05). The luciferase activity of GLSTAT3-Lu in the shTIRC7-1 and shTIRC7-2 groups was decreased compared with the sh-control and blank control groups (P<0.05); there was no significant difference in the luciferase activity of GLSTAT3-Lu between the shTIRC7-1 and shTIRC7-2 groups (P>0.05).
Figure 2.
Changes in STAT3 expression and activity after downregulation of TIRC7. (A) Changes in STAT3 luciferase activity after downregulation of TIRC7. (B) Changes in STAT3 protein expression and phosphorylation after downregulation of TIRC7. TIRC7, T-cell immune response cDNA 7; sh(RNA), short hairpin (RNA); p, phosphorylated.
Effects of TIRC7 knockdown on the proliferation, apoptosis and differentiation of CD4+ T cells
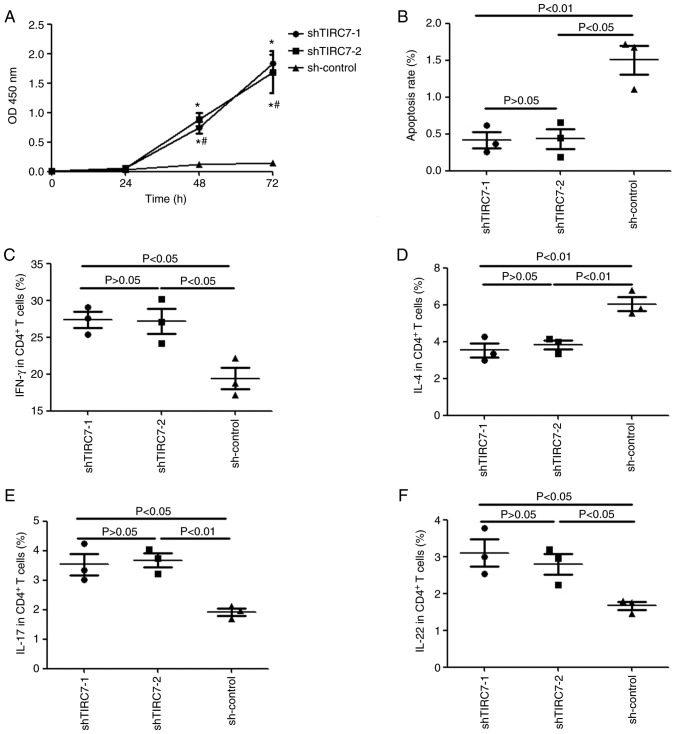
A CCK-8 assay was used to monitor the proliferation of CD4+ T cells from patients with acute GVHD. Fig. 3A indicated that there was no significant difference in the optical density (OD)450 nm value for the shTIRC7-1, shTIRC7-2 and sh-control groups after CD4+ T cells were cultured for 24 h (P>0.05); however, at 48 and 72 h, the OD450 nm value in the shTIRC7-1 and shTIRC7-2 groups was markedly higher than that in the sh-control group, particularly at 72 h (P<0.05). However, there was no significant difference in the OD450 nm value for the shTIRC7-1 and shTIRC7-2 groups at 48 and 72 h (P>0.05).
Figure 3.
Changes in the proliferation, apoptosis and differentiation of CD4+ T cells after downregulation of TIRC7. (A) Changes in the proliferation of CD4+ T cells after downregulation of TIRC7. (B) Changes in the apoptosis of CD4+ T cells after downregulation of TIRC7. Changes in the secretion of (C) IFN-γ, (D) IL-4, (E) IL-17 and (F) IL-22 after downregulation of TIRC7. *P<0.05 vs. sh-control; #P>0.05 vs. sh-TIRC7-1. TIRC7, T-cell immune response cDNA 7; sh(RNA), short hairpin (RNA); IL, interleukin; IFN, interferon.
Annexin V and 7-AAD were selected to label apoptotic CD4+ T cells. The apoptotic cell rates of CD4+ T cells in the shTIRC7-1, shTIRC7-2 and sh-control groups were 0.42±0.11%, 0.43±0.14% and 1.50±0.20%, respectively; the apoptosis rates of CD4+ T cells in the shTIRC7-1 and shTIRC7-2 groups were both significantly lower than that in the sh-control group (P<0.01 and P<0.05, respectively; Fig. 3B).
IFN-γ, IL-4, IL-17 and IL-22 levels in the CD4+ T cells from patients with acute GVHD were monitored via flow cytometry after TIRC7 was downregulated. As presented in Fig. 3C, E and F, after TIRC7 was downregulated, compared with those in the sh-control group, the levels of IFN-γ, IL-17 and IL-22 in CD4+ T cells in the shTIRC7-1 and shTIRC7-2 groups were all elevated (P<0.05); however, IL-4 levels in the shTIRC7-1 and shTIRC7-2 groups were instead downregulated (P<0.01; Fig. 3D). No significant differences in IFN-γ, IL-4, IL-17 and IL-22 levels were observed between the shTIRC7-1 and shTIRC7-2 groups (P>0.05).
Survival of mice post-transplantation
In the acute GVHD mice model, CTLA-4 and TIRC7 monoclonal antibodies were both administered via intraperitoneal injection. The present study monitored the levels of CTLA-4 and TIRC7 in recipient mice on day 21 after transplantation, and revealed that compared with the control group, CTLA-4 plasma levels and relative expression in each experimental group were decreased, whereas TIRC7 levels were elevated. Compared with the mice that were solely administered TIRC7 antibody post-allogeneic bone marrow transplant (allo-BMT), the mice co-administered with CTLA-4 and TIRC7 monoclonal antibody exhibited no significant difference in the expression of TIRC7; nevertheless, the expression of CTLA-4 in the CTLA-4-alone group was lower than that in the CTLA-4/TIRC7 co-administration group (data not shown). The aforementioned data suggested successful manipulation of the expression of TIRC7 or CTLA-4 in mice with acute GVHD after transplantation.
Overall survival of mice after transplantation was monitored. Table I demonstrates that the mice in the transplantation control group were all alive, whereas the mice in the TBI group all died between 5 and 15 days after allo-BMT, with a median survival time of 8 days. The median survival time of the A1-A4 groups was 18.0, 27.5, 28.5 and 32.0, and long-term survival rates were 50, 60, 60 and 70%, respectively. The median survival time of the A4 group was significantly longer than that of the A1, A2 and A3 groups (P<0.01, P<0.05 and P<0.05, respectively). The median survival times of the B1-B4 groups were 14.0, 20.5, 20.5 and 25.0 days, and long-term survival rates were 10, 20, 20 and 40%, respectively. The median survival time of the B4 group was significantly longer than that of the B1, B2 and B3 groups (P<0.01, P<0.05 and P<0.05, respectively). In the experimental groups, the long-term survival rate of the A4 group was the highest, which was higher than that of the B4 group (P<0.01).
Table I.
Survival of mice after allogeneic bone marrow transplant.
| Group | Median survival time, days | Long-term survival rate, % |
|---|---|---|
| TBI group | 8.00 | 0 |
| A1 group | 18.00a,d | 50 |
| A2 group | 27.50b,c | 60 |
| A3 group | 28.50b,c | 60 |
| A4 group | 32.00b | 70 |
| B1 group | 14.00a | 10 |
| B2 group | 20.50b,e | 20 |
| B3 group | 20.50b,e | 20 |
| B4 group | 25.00b | 40 |
P<0.05
P<0.01 vs. TBI
P<0.05
P<0.01 vs. A4
P<0.05 vs. B4. TBI, total body irradiation.
Changes in body weight of recipient mice
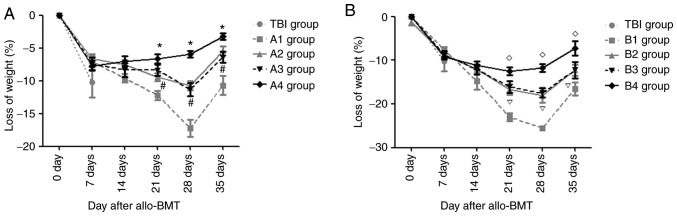
From day 4 post-transplantation, the recipient mice gradually exhibited decreased activity, ate less and exhibited decreased body weight. On day 7 after allo-BMT, there was no significant difference in the magnitude of weight loss between each experimental group and the TBI group (P>0.05). However, during the following periods, there were different degrees of improvement in the body weights of mice in the experimental groups.
Fig. 4A demonstrates that on day 28 post-transplantation, the mean body weights of mice in the A1-A3 groups reached their lowest values, following which body weights began to rise (this does not control for animals that were sacrificed due to excessive weight loss); however, in the A4 group, the minimum body weight of mice was observed on day 14. On days 7 and 14 post-allo-BMT, there was no significant difference in the magnitude of weight loss in the A1-A4 groups (P>0.05). On days 21, 28 and 35 post-allo-BMT, the magnitude of weight loss in the A4 group was the lowest compared with that in the A1-A3 groups, with lower weight loss in the A2 and A3 groups than that in the A1 group (all P<0.05); however, there was no significant difference between the A2 and A3 groups (P>0.05). The body weights of mice in the B1-B3 groups reached their lowest point on day 28 post-transplantation, then began to rise; however, in the B4 group, the minimum body weight of mice was observed on day 14.
Figure 4.
Changes in body weight loss in the experimental groups and TBI group after transplantation. (A) Body weight loss in A1-A4 groups and TBI group after transplantation. (B) Body weight loss in B1-B4 groups and TBI group after transplantation. *P<0.05 vs. A2 group; #P>0.05 vs. A3 group; #P<0.05 vs. B2 group; P>0.05 vs. B3 group. TBI, total body irradiation; allo-BMT, allogeneic bone marrow transplant.
In the B group, on days 21, 28 and 35 post-allo-BMT, the magnitude of weight loss in the B4 group was the lowest, but was lower in the B2 and B3 groups compared with in the B1 group (all P<0.05). There was no significant difference in the magnitude of weight loss between the B2 and B3 groups (P>0.05; Fig. 4B).
Recovery of hematopoietic reconstitution in recipient mice
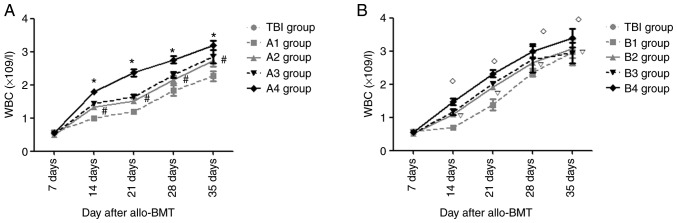
On day 7 post- transplantation, the mean leukocyte numbers in each group were lowest, without any significant differences compared with the other experimental groups (P>0.05).
Fig. 5A demonstrates that on days 14, 21, 28 and 35 post-allo-BMT, compared with the A1 group, the mean leukocyte counts in the A2-A4 groups were elevated (P<0.05). The mean leukocyte count in the A4 group was the highest compared with that in the A1-A3 groups; however, there was no significant difference between the A2 and A3 groups (P>0.05). Compared with the B1 group, the mean leukocyte levels in the B2-B4 groups were elevated on days 14, 21, 28 and 35 after transplantation (P<0.05), with the highest mean leukocyte number in the B4 group; however, there was no significant difference between the B2 and B3 groups (P>0.05; Fig. 5B).
Figure 5.
Hematopoietic reconstitution in experimental groups and TBI group after transplantation. (A) Hematopoietic reconstitution in A1-A4 groups and TBI group after transplantation. (B) Hematopoietic reconstitution in B1-B4 groups and TBI group after transplantation. *P<0.05 vs. A2 group; #P>0.05 vs. A3 group; #P<0.05 vs. B2 group; P>0.05 vs. B3 group. TBI, total body irradiation; allo-BMT, allogeneic bone marrow transplant; WBC, white blood cell.
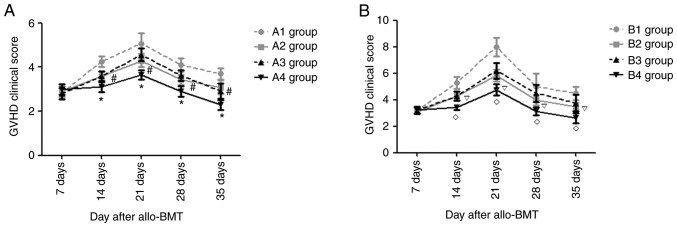
Changes in acute GVHD clinical scores
After transplantation, the recipient mice in each experimental group exhibited different degrees of acute GVHD symptoms, such as anorexia, weight loss, ruffled fur and diarrhea. At days 14, 21, 28 and 35 post-allo-BMT, there were notable differences in the degree of diarrhea, weight loss and hunched posture in the A and B groups. The present study semi-quantitatively scored the clinical manifestations of recipient mice in each experimental group after transplantation and, as presented in Fig. 6, at the indicated time points, the acute GVHD clinical scores in the mice of group B were higher than those in group A (P<0.05). In the mice from group A, the acute GVHD clinical score in the mice of the A4 group was the lowest (P<0.05), and there was no significant difference in acute GVHD clinical score between the A2 and A3 groups (P>0.05). At days 14, 21, 28 and 35 after transplantation, the acute GVHD clinical scores in the B4 group were the lowest (P<0.05) and those in the B1 group were the highest (P<0.05), but there was no significant difference in the acute GVHD clinical scores in the B2 and B3 groups (P>0.05).
Figure 6.
Changes in acute GVHD clinical scores in experimental groups after transplantation. (A) Changes of acute GVHD clinical scores in A1-A4 groups after transplantation. (B) Changes of acute GVHD clinical scores in B1-B4 groups after transplantation. *P<0.05 vs. A2 group; #P>0.05 vs. A3 group; #P<0.05 vs. B2 group; P>0.05 vs. B3 group. GVHD, graft-vs.-host disease; allo-BMT, allogeneic bone marrow transplant.
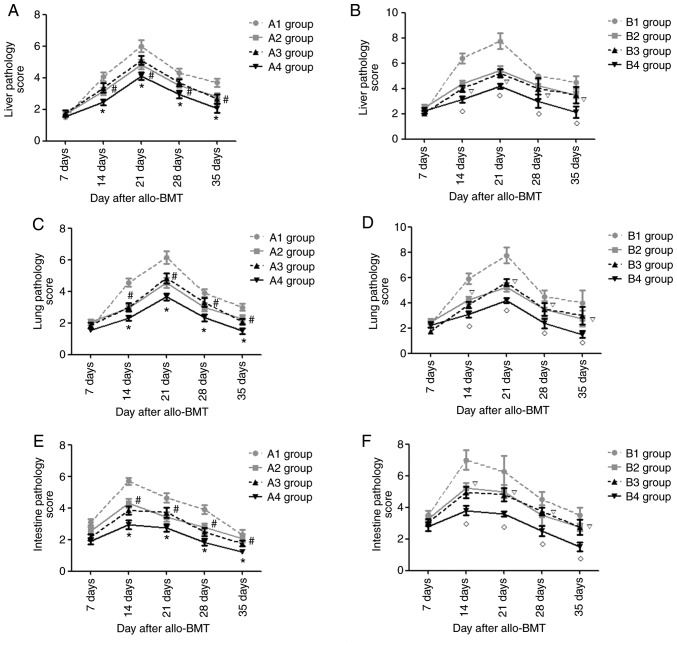
Pathological changes in targeted organs (liver, lung, colon) after allo-BMT
Starting from day 7 post-transplantation, the recipient mice in the experimental groups exhibited liver pathology suggesting acute GVHD, such as liver cell edema and periportal inflammatory cell infiltration. During the following period, the severity of the liver pathology increased and peaked at day 21 post-allo-BMT, before gradually decreasing. As presented in Figs. 7A and B, and S1 and S2, the present study semi-quantitatively scored the liver pathology of recipient mice in each experimental group and revealed that on days 14, 21, 28 and 35 post-transplantation, the acute GVHD scores of the liver in the A4 group were lowest compared with those in the A1-A3 groups (P<0.05) and the acute GVHD scores in the A1 groups were the highest in the A group (P<0.05). There was no significant difference in the acute GVHD scores of the liver in the A2 and A3 groups (P>0.05). At the indicated time points, the acute GVHD scores of the liver in the B4 group were the lowest of B groups (P<0.05) and there was no significant difference between the B2 and B3 groups (P>0.05). In each experimental group, on day 21 post-transplantation, the acute GVHD scores of the liver in the A4 group were the lowest (P<0.05); however, there were no significant differences in the acute GVHD scores in the A4 and B4 groups on days 14, 28 and 35 after allo-BMT (P>0.05).
Figure 7.
Pathological liver/lung/colon changes in experimental groups after transplantation. Pathological liver changes in (A) A1-A4 groups and (B) B1-B4 groups after transplantation. Pathological lung changes in (C) A1-A4 groups and (D) B1-B4 groups after transplantation. Pathological colon changes in (E) A1-A4 groups and (F) B1-B4 groups after transplantation. *P<0.05 vs. A2 group; #P>0.05 vs. A3 group; #P<0.05 vs. B2 group; P>0.05 vs. B3 group. Allo-BMT, allogeneic bone marrow transplant.
Similarly, the recipient mice in the experimental groups exhibited lung/colon pathology suggesting acute GVHD, such as liver cell edema, periportal inflammatory cell infiltration and necrotic cells in crypts, and infiltration of the lamina propria. The severity of the lung pathology increased and peaked on day 21 post-allo-BMT, whereas it peaked at day 14 in the colon and then gradually decreased. As presented in Figs. 7C-F and S3–S6, it was revealed that at days 14, 21, 28 and 35 post-transplantation, the acute GVHD scores of lung/colon in the A4 group were lowest compared with those in the A1-A3 groups (P<0.05) and the acute GVHD scores in the A1 group were highest in the A group (P<0.05). There was no significant difference in the acute GVHD scores of the lung/colon in the A2 and A3 groups (P>0.05). At the indicated time points, the acute GVHD scores of the lung/colon in the B4 group were lowest of the experimental B groups (P<0.05) and there was no significant difference between the B2 and B3 groups (P>0.05).
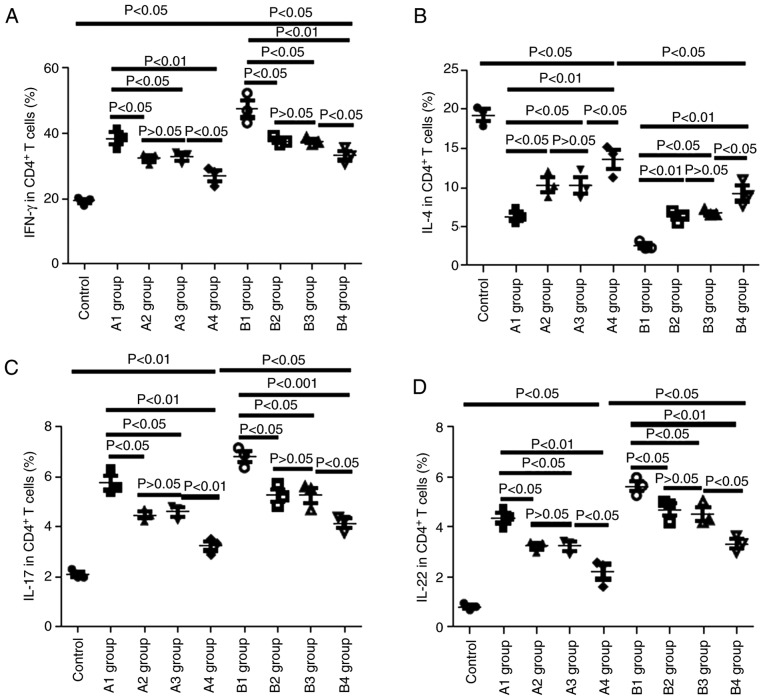
Changes in Th cells in recipient mice
On day 21 post-allo- BMT, the present study collected the peripheral blood of recipient mice and monitored the levels of Th cells via flow cytometry. As presented in Fig. 8, compared with the control group, the levels of IFN-γ in the A and B groups were markedly elevated, and IFN-γ levels in the B group were higher than those in the corresponding A group; for example, IFN-γ levels in the B1 group were higher than those in the A1 group (P<0.05), IFN-γ levels in the B2 group were higher than those in the A2 group (P<0.05), and so on. The levels of IFN-γ in the A1 group were higher than those in the A2-A4 groups (P<0.05, P<0.05 and P<0.01, respectively) and there was no significant difference in the IFN-γ levels between the A2 and A3 groups (P>0.05). In the recipient mice of the B group, IFN-γ levels in the B4 group were the lowest (P<0.05) and there was no significant difference in the IFN-γ levels between the B2 and B3 groups (P>0.05).
Figure 8.
Changes in cytokines in recipient mice on day 21 post-allo-BMT. (A) Changes in IFN-γ-positive Th1 in recipient mice on day 21 post-allo-BMT as determined via flow cytometry. (B) Changes in IL-4-positive Th2 cells in recipient mice on day 21 post-allo-BMT as determined via flow cytometry. (C) Changes in IL-17-positive Tg17 cells in recipient mice on day 21 post-allo-BMT as determined via flow cytometry. (D) Changes in IL-22-positive Th22 cells in recipient mice on day 21 post-allo-BMT as determined via flow cytometry. Allo-BMT, allogeneic bone marrow transplant; Th, T helper; IFN, interferon; IL, interleukin.
As presented in Fig. 8C and D, similar results were observed for IL-17 and IL-22 levels as were observed for IFN-γ. Conversely, inverse patterns were observed for IL-4 levels. In the recipient mice of the A group, IL-4 levels in the A4 group were the highest (P<0.05), with those in the A1 group lowest (P<0.05); there was no significant difference in IL-4 levels between the A2 and A3 groups (P>0.05). In the recipient mice of the B group, IL-4 levels in the B4 group were the highest (P<0.05) and there was no significant difference in IL-4 levels between the B2 and B3 groups (P>0.05).
Discussion
TIRC7 has been identified to be critical in T cell activation (9,18); however, the role of TIRC7 in acute GVHD remains unclear. It has previously been demonstrated that TIRC7 levels in patients with acute GVHD were higher than healthy controls, and were also markedly declined following treatment, suggesting that TIRC7 level may be an indicator to evaluate the response of patients with acute GVHD to treatment (8). It has been demonstrated that CTLA-4 may play a negative role in the regulation of acute GVHD (6,7). The present study also demonstrated that CTLA-4 may be involved in the pathogenesis of acute GVHD, and that it may downregulate Th1 cell levels by increasing the expression of STAT3 in acute GVHD (19); meanwhile, other studies have reported that TIRC7 is the upstream regulatory molecule of CTLA-4 (9,11). Therefore, the present study hypothesized that both TIRC7 and CTLA-4 play important roles in acute GVHD, and that TIRC7 may regulate the expression of CTLA-4 in acute GVHD. From the results of the present study, it was revealed that after TIRC7 expression was knocked down, the expression of CTLA-4 was decreased; however, after CTLA-4 expression was increased, the expression of TIRC7 was not changed, which supported the hypothesis that TIRC7 was the upstream molecule of CTLA-4. According to the previous studies, the intracellular regions of TIRC7 and CTLA-4 both contain immunoreceptor tyrosine-based inhibitory motifs (ITIMs); thus, TIRC7 plays a positive role in the regulation of CTLA-4 expression in other animal models (9,20). This may contradict the present findings that when acute GVHD occurred, the level of TIRC7 was elevated, but CTLA-4 levels were decreased (data not shown). This may be because CTLA-4 is a member of the immunoglobulin superfamily, which is mainly located on the surface of Th cells, and the numbers of Th cells decrease after acute GVHD occurs. Although the activity of CTLA-4 on the surface of a single Th cell is enhanced, the overall expression of CTLA-4 was downregulated.
Numerous studies have confirmed that the JAK/STAT3 pathway plays an important role in the occurrence and development of acute GVHD (21,22). Dendritic cells could increase STAT3 expression and decrease the severity of acute GVHD in mouse models by inhibiting the secretion of cytokines such as IFN-α and IL-12 (23). Ma et al (21) revealed that STAT3 could affect the secretion of IL-17 and other inflammatory cytokines, and decrease the severity of acute GVHD by regulating the expression levels of downstream molecules, such as NF-κB and MAPK. In the present study, dual-luciferase reporter gene and western blot assays were utilized to monitor the levels of STAT3 phosphorylation. After cells were transfected with pGPU6-shTIRC7, STAT3 luciferase reporter gene plasmid luciferase activity was markedly decreased, as were the levels of STAT3 phosphorylation. Meanwhile, the activation of T lymphocytes was enhanced, and the degree of apoptosis in T cells was decreased with increased secretions of IFN-γ and other cytokines. Increased levels of IFN-γ, IL-17 and IL-22, and decreased IL-4 levels were observed in the A and B groups, indicating an imbalance of Th1/17/22 and Th2 cells in the pathogenesis of GVHD, consistent with a previous study reporting that T cell activation was remarkably inhibited, with reduced levels of IFN-γ, IL-17 and IL-22 (19). From the in vitro results in the present study, it was indicated that TIRC7 upregulated the expression of CTLA-4, increased the activation of STAT3, inhibited the proliferation of T cells, promoted the apoptosis of T cells and decreased the secretion of cytokines.
Establishing appropriate animal models that effectively simulate or replicate clinical diseases can aid with understanding the mechanisms underlying the clinical disease. Therefore, in order to clarify the specific role of TIRC7 in acute GVHD, the present study established a mouse model for acute GVHD with different severities. According to previous studies (24,25), the severity of acute GVHD is dependent on the splenic lymphocytes from donor mice. When the splenic lymphocytes were 5×106/mouse, the degree of acute GVHD was moderate-to-severe. TIRC7 and CTLA-4 monoclonal antibodies were administered alone or in combination into the recipient mice post-allo-BMT, and the changes in acute GVHD severity levels were observed by clinical scores, histopathological examination and other indicators.
According to the results in vivo, TIRC7 or CTLA-4 monoclonal antibody intraperitoneally injected could effectively decrease the severity of acute GVHD and promote hematopoietic reconstitution; the two antibodies had an additive effect. Referring to other previous studies (9,26) and preliminary experiments, the optimal dose of CTLA-4 antibody was selected as 40 µg/mouse, to be administered at day 0 post-transplantation; the optimal dose of TIRC7 antibody was 25 µg/mouse administered on days 0, 1, 2, 3, 4 and 7 post-allo-BMT. The potential basis of the additive effect of the two antibodies was hypothesized to involve actitation of intracellular ITIM (27) districts in both molecules following co-administration, which then negatively regulated the levels of T cells. Thus, the severity of acute GVHD was effectively decreased. The average survival time of mice, acute GVHD clinical scores and pathology scores in the experimental groups supported this conclusion. TIRC7 and CTLA-4 antibodies also promoted hematopoietic reconstitution; however, the mechanism was not clear and requires further investigation.
In summary, through in vitro and in vivo experiments, the present study revealed that TIRC7 could positively regulate CTLA-4 expression, upregulate the activity of STAT3, inhibit the activation of T cells and cytokine secretion, and subsequently modulate the development and progression of acute GVHD. The present study may deepen the understanding of the pathogenesis of acute GVHD and provide novel approaches for controlling acute GVHD.
Supplementary Material
Acknowledgments
We acknowledge Professor Xiu-Ying Pan, Professor Xu-Peng He, Dr De-Peng Li and Professor Yi-Hong Huang (all Department of Hematology, The Affiliated Hospital of Xuzhou Medical University) for their clinical assistance to patients included in this study.
Funding
This work was supported by grants from the National Nature Science Foundation of China (grant nos. 81300377, 81300441, 81700179 and 81600145), the Nature Science Foundation of Jiangsu Province (grant nos. BK20160232 and BK20160226), China Postdoctoral Science Foundation funded project (grant nos. 2015113010 and 2016M590508), the Foundation of Jiangsu Province Six Talents Peak (grant no. 2015-WSW-058), the Foundation of Jiangsu Province Six-one Project (grant no. LGY2018084) and the Clinical Technology Backbone Training Program of Xuzhou City (grant no. 2018GG002).
Availability of data and materials
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
FZ, TQ and SZ designed the study and wrote the manuscript. KZ, CC and JQ primarily performed the experiments, wrote the manuscript and prepared the figures. BP, ZY, WC and QL were involved in performing the experiments. QW, JC and WS made substantial contributions to the acquisition and analysis of data. LZ, HS, ZL and KX made contributions to the analysis and interpretation of data, and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Ethical approval for this study was obtained from the Medical Ethics Committee of the Affiliated Hospital of Xuzhou Medical University. Patients provided written informed consent prior to sample collection. Animal experiments were approved by the Animal Committee of Xuzhou Medical University and all protocols were performed in accordance with Institutional Animal Care and Use Committee guidelines.
Patient consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing interests.
References
- 1.Ammer J, Prantl L, Holler B, Landfried K, Wolff D, Karrer S, Andreesen R, Holler E. Successful treatment of a refractory skin ulcer in chronic cutaneous GVHD after allogeneic HSCT with split-thickness skin allografting from the stem cell donor. Bone Marrow Transplant. 2012;47:1368–1369. doi: 10.1038/bmt.2012.16. [DOI] [PubMed] [Google Scholar]
- 2.Chen X, Chang CH, Stein R, Goldenberg DM. The humanized anti-HLA-DR moAb, IMMU-114, depletes APCs and reduces alloreactive T cells: Implications for preventing GVHD. Bone Marrow Transplant. 2012;47:967–980. doi: 10.1038/bmt.2011.203. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai HY, Chou TY, Tzeng CH, Lee OK. Cytokine profiles in various graft-versus-host disease target organs following hematopoietic stem cell transplantation. Cell Transplant. 2012;21:2033–2045. doi: 10.3727/096368912X653110. [DOI] [PubMed] [Google Scholar]
- 5.Ewing MM, Karper JC, Abdul S, de Jong RC, Peters HA, de Vries MR, Redeker A, Kuiper J, Toes RE, Arens R, et al. T-cell co-stimulation by CD28-CD80/86 and its negative regulator CTLA-4 strongly influence accelerated atherosclerosis development. Int J Cardiol. 2013;168:1965–1974. doi: 10.1016/j.ijcard.2012.12.085. [DOI] [PubMed] [Google Scholar]
- 6.Cho H, Chung YH. Construction and in vitro and in vivo analyses of tetravalent immunoadhesins. J Microbiol Biotechnol. 2012;22:1066–1076. doi: 10.4014/jmb.1201.01026. [DOI] [PubMed] [Google Scholar]
- 7.Yoo JS, Lee YJ, Yoon JW, Hyung KE, Hwang KW. CTLA-4-Tg/CD-28-KO mice exhibit reduced T cell proliferation in vivo compared to CD-28-KO mice in a graft-versus-host disease model. Korean J Physiol Pharmacol. 2012;16:349–353. doi: 10.4196/kjpp.2012.16.5.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu F, Qiao J, Chen W, Pan B, Wu QY, Cao J, Sang W, Yan ZL, Zeng LY, Li ZY, Xu KL. Increased expression of T cell immune response cDNA 7 in patients with acute graft-versus-host disease. Ann Hematol. 2015;94:1025–1032. doi: 10.1007/s00277-015-2300-8. [DOI] [PubMed] [Google Scholar]
- 9.Kumamoto Y, Tomschegg A, Bennai-Sanfourche F, Boerner A, Kaser A, Schmidt-Knosalla I, Heinemann T, Schlawinsky M, Blumberg RS, Volk HD, Utku N. Monoclonal antibody specific for TIRC7 induces donor-specific anergy and prevents rejection of cardiac allografts in mice. Am J Transplant. 2004;4:505–514. doi: 10.1111/j.1600-6143.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- 10.Utku N, Boerner A, Tomschegg A, Bennai-Sanfourche F, Bulwin GC, Heinemann T, Loehler J, Blumberg RS, Volk HD. TIRC7 deficiency causes in vitro and in vivo augmentation of T and B cell activation and cytokine response. J Immunol. 2004;173:2342–2352. doi: 10.4049/jimmunol.173.4.2342. [DOI] [PubMed] [Google Scholar]
- 11.Kumamoto Y, Tamura A, Volk HD, Reinke P, Löhler J, Tullius SG, Utku N. TIRC7 is induced in rejected human kidneys and anti-TIRC7 mAb with FK506 prolongs survival of kidney allografts in rats. Transpl Immunol. 2006;16:238–244. doi: 10.1016/j.trim.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 12.Zhu F, Qiao JL, Wu QY, Cao J, Zeng LY, Li ZY, Xu KL. Elevated levels of T-cell immune response cDNA 7 in patients with immune thrombocytopenia. Hematology. 2014;19:477–482. doi: 10.1179/1607845414Y.0000000156. [DOI] [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Zhu F, Qiao J, Zhong XM, Wu QY, Chen W, Yao Y, Niu MS, Fu CL, Zeng LY, Li ZY, Xu KL. Antithymocyte globulin combined with cyclosporine A down-regulates T helper 1 cells by modulating T-cell immune response cDNA 7 in aplastic anemia. Med Oncol. 2015;32:197. doi: 10.1007/s12032-015-0647-2. [DOI] [PubMed] [Google Scholar]
- 15.Cooke KR, Kobzik L, Martin TR, Brewer J, Delmonte J, Jr, Crawford JM, Ferrara JL. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I the roles of minor H antigens and endotoxin. Blood. 1996;88:3230–3239. doi: 10.1182/blood.V88.8.3230.bloodjournal8883230. [DOI] [PubMed] [Google Scholar]
- 16.Blazar BR, Taylor PA, McElmurry R, Tian L, Panoskaltsis- Mortari A, Lam S, Lees C, Waldschmidt T, Vallera DA. Engraftment of severe combined immune deficient mice receiving allogeneic bone marrow via In utero or postnatal transfer. Blood. 1998;92:3949–3959. doi: 10.1182/blood.V92.10.3949. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan DH, Anderson BE, McNiff JM, Jain D, Shlomchik MJ, Shlomchik WD. Target antigens determine graft-versus-host disease phenotype. J Immunol. 2004;173:5467–5475. doi: 10.4049/jimmunol.173.9.5467. [DOI] [PubMed] [Google Scholar]
- 18.Utku N, Heinemann T, Winter M, Bulwin CG, Schlawinsky M, Fraser P, Nieuwenhuis EE, Volk HD, Blumberg RS. Antibody targeting of TIRC7 results in significant therapeutic effects on collagen-induced arthritis in mice. Clin Exp Immunol. 2006;144:142–151. doi: 10.1111/j.1365-2249.2006.03044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu F, Zhong XM, Qiao J, Liu Q, Sun HY, Chen W, Zhao K, Wu QY, Cao J, Sang W, et al. Cytotoxic T lymphocyte antigen-4 down-regulates T helper 1 cells by increasing expression of signal transducer and activator of transcription 3 in acute graft-versus-host disease. Biol Blood Marrow Transplant. 2016;22:212–219. doi: 10.1016/j.bbmt.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Bulwin GC, Heinemann T, Bugge V, Winter M, Lohan A, Schlawinsky M, Schulze A, Wälter S, Sabat R, Schülein R, et al. TIRC7 inhibits T cell proliferation by modulation of CTLA-4 expression. J Immunol. 2006;177:6833–6841. doi: 10.4049/jimmunol.177.10.6833. [DOI] [PubMed] [Google Scholar]
- 21.Ma HH, Ziegler J, Li C, Sepulveda A, Bedeir A, Grandis J, Lentzsch S, Mapara MY. Sequential activation of inflammatory signaling pathways during graft-versus-host disease (GVHD): Early role for STAT1 and STAT3. Cell Immunol. 2011;268:37–46. doi: 10.1016/j.cellimm.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Betts BC, Sagatys EM, Veerapathran A, Lloyd MC, Beato F, Lawrence HR, Yue B, Kim J, Sebti SM, Anasetti C, Pidala J. CD4+ T cell STAT3 phosphorylation precedes acute GVHD, and subsequent Th17 tissue invasion correlates with GVHD severity and therapeutic response. J Leukoc Biol. 2015;97:807–819. doi: 10.1189/jlb.5A1114-532RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Capitini CM, Nasholm NM, Chien CD, Larabee SM, Qin H, Song YK, Klover PJ, Hennighausen L, Khan J, Fry TJ. Absence of STAT1 in donor-derived plasmacytoid dendritic cells results in increased STAT3 and attenuates murine GVHD. Blood. 2014;124:1976–1986. doi: 10.1182/blood-2013-05-500876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu Y, Wang D, Liu C, Kaosaard K, Semple K, Anasetti C, Yu XZ. Prevention of GVHD while sparing GVL effect by targeting Th1 and Th17 transcription factor T-bet and RORγt in mice. Blood. 2011;118:5011–5020. doi: 10.1182/blood-2011-03-340315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varkila K, Hurme M. Reduction of acute graft-versus-host disease-related mortality and cytotoxic T lymphocyte induction after pretreatment of the recipient with anti-asialo GM1 antibody in the murine P-to-F1 model. Transplant Proc. 1987;19:2690–2691. [PubMed] [Google Scholar]
- 26.Krummel MF, Sullivan TJ, Allison JP. Superantigen responses and co-stimulation: CD28 and CTLA-4 have opposing effects on T cell expansion in vitro and in vivo. Int Immunol. 1996;8:519–523. doi: 10.1093/intimm/8.4.519. [DOI] [PubMed] [Google Scholar]
- 27.Sarmay G, Koncz G, Pecht I, Gergely J. Fc gamma receptor type IIb induced recruitment of inositol and protein phosphatases to the signal transductory complex of human B-cell. Immunol Lett. 1997;57:159–164. doi: 10.1016/S0165-2478(97)00055-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.