Abstract
Objectives
Describe pediatric palliative care (PPC) consult in children with heart disease; retrospectively apply Center to Advance Palliative Care (CAPC) criteria for PPC consults; determine the impact of PPC on end of life.
Design
A retrospective single-center study
Setting
A 16-bed cardiac intensive care unit (CICU), in a university-affiliated tertiary care children’s hospital.
Patients
Children (aged 0–21years) with heart disease admitted to the CICU from January 2014 to June 2017.
Measurements and Results
Over one thousand (n=1, 389) patients were admitted to the CICU with 112 (8%) receiving a PPC consultation. Patients who received a consult were different from those who did not. Patients who received PPC were younger at first hospital admission (median 63 days vs. 239 days; p =0.003), had a higher median number of complex chronic conditions (CCCs) at the end of first hospitalization (3 vs.1; p<0.001), longer cumulative length of stay in the CICU (11 days vs.2d; p<0.001) and hospital (60 days vs. 7d; p<0.001) and higher mortality rates (38% vs. 3%; p <0.001). When comparing location and modes of death, patients who received PPC were more likely to die at home (24% vs. 2%; p=0.02) and had more comfort care at the end of life (36% vs. 2%; p=0.002) compared to those who did not. The CAPC guidelines identified 158 patients who were eligible for PPC consultation; however, only 30 patients (19%) in our sample received a consult.
Conclusions
PPC consult rarely occurred in the CICU. Patients who received a consult were medically complex and experienced high mortality. Comfort care at the end of life and death at home was more common when PPC was consulted. Missed referrals were apparent when CAPC criteria were retrospectively applied.
Keywords: pediatric palliative care, children with heart disease, complex chronic conditions, cardiac intensive care unit, comfort care, end of life care
Pediatric palliative care (PPC) improves the quality of life of patients and families who face life-threatening illness (1, 2). The PPC team provides support to families over the patient’s disease trajectory as well as the end of life (3, 4). PPC involvement has been associated with fewer deaths occurring within the intensive care unit (ICU) and during active resuscitation attempts (5, 6). Furthermore, PPC is associated with shorter hospital and ICU stays (7, 8) and fewer invasive interventions (9–13). In the last decade, the American Academy of Pediatrics (AAP) and the Improving Palliative Care in the Intensive Care Unit Advisory Board (IPA-ICU) recognize the importance of palliative care and recommend involvement early in the disease course for children with complex chronic health issues, life-threatening, or terminal conditions (12, 13).
Integration of palliative care delivery into ICUs is rapidly becoming the standard for high quality care of critically ill children (11, 13). As the mortality rates for pediatric heart surgery continue to decline, the survivors are at risk for premature death due to their heart disease and coexisting chronic conditions, which often result in frequent hospitalizations and medical interventions (14–16). This highlights the need for PPC in this group of patients. Despite the benefits of PPC in children with life-limiting conditions such as heart disease, rates of PPC referral remain low (9) and there is a paucity of literature describing the use of PPC in this population.
Our study aim was to compare the characteristics of patients admitted to a cardiac intensive care unit (CICU) with heart disease and examine differences between children who received PPC to those who did not. We hypothesized that PPC occurs more often in patients with higher clinical disease severity, more complex conditions, and lower survival compared to patients who did not receive PPC. Further, we hypothesized that there are missed opportunities for PPC referral according to the Center to Advance Palliative Care (CAPC) guidelines (17).
MATERIALS AND METHODS
This is a retrospective cohort study of patients with cardiac disease admitted to the CICU at Primary Children’s Hospital. The Institutional Review Boards at the University of Utah and Primary Children’s Hospital approved this study.
Study Setting
Primary Children’s Hospital is a freestanding 289-bed academic children’s hospital in Salt Lake City, Utah. The CICU has 16 beds and treats approximately 400 children undergoing open-heart surgery each year.
The interdisciplinary PPC team includes board-certified Pediatric Hospice and Palliative Medicine physicians, nurse practitioners, a social worker, a registered nurse, and a chaplain. Any child with a chronic, potentially life-limiting medical condition is eligible for a PPC consult. While there is no automatic referral process at our institution, licensed medical providers in any hospital unit may place a referral for PPC consult. The patient or family can accept or refuse a PPC consult. Once a consult has occurred, the palliative care team remains available to support the family until the condition has resolved, the patient has transitioned to medical care for adults, or the patient dies.
Participants
Patients aged 0– 21 years diagnosed with heart disease and admitted to the CICU between January 2014 to June 2017.
Measurements
We queried the institutional data warehouse to determine receipt of PPC consult, patient demographics, medical history including heart disease treatment and length of stay, disease severity measures, mode and location of death (when applicable). We divided the cohort into two groups depending on the presence or absence of a PPC consult.
Complex chronic conditions (CCCs), hospital (HLOS) and CICU length of stay (CICU LOS), Risk Adjustment for Congenital Heart Surgery (RACHS) and mortality were used as indicators of disease severity. Patients were categorized based on the treatment of their heart disease as surgical, medical, or cardiac catheterization.
Patients were identified as having CCCs based on codes from the International Classification of Disease version 10 Clinical Modification (ICD-10-CM) and as defined by Feudtner and colleagues (18). The number and type of CCCs were determined for all patients and for patients who died. The presence of CCCs was identified at two time points: 1) at the end of first hospitalization and 2) at the end of study or time of death.
The RACHS is a classification of surgical procedures into six risk categories from 1 (least complex) to 6 (most complex) to compare in-hospital mortality for children undergoing surgery for congenital heart disease (19). If a patient had a surgical repair and a cardiac catheterization, the patient was classified as surgical. We categorized RACHS groups into 1–2, 3–4, and 5–6. For patients with more than one surgery, the highest RACHS score during the study period was reported.
Modes of death were classified into five categories by the care provided at the time of death; 1) withdrawal of life-sustaining therapies defined as discontinuation of mechanical ventilation, blood pressure supporting infusions, and extracorporeal membrane oxygenation; 2) no-escalation of therapy defined as withholding new therapies while continuing current ones; 3) comfort care defined as symptom management either in the hospital, hospice or chronic care facility; 4) died during cardiopulmonary resuscitation (CPR) defined as patient arrested and underwent CPR without success at home or during hospitalization; 5) unknown (6,20).
Retrospective Application of the Center to Advance Palliative Care (CAPC) Referral Criteria
The CAPC criteria include over 15 medical conditions by organ systems. For cardiac patients the criteria include, but are not limited to, single ventricle physiology, cardiomyopathy, myocarditis, cardiac transplant, and a combination of cardiac diagnosis with underlying neurological and congenital/genetic diagnosis (17). We applied the CAPC criteria to the patients in the RACHS 5–6 group and patients with heart disease who also had combined neurological and congenital/genetic CCCs in order to ascertain the number of patients that would have qualified for PPC consult if the CAPC criteria had been followed.
Statistical Analysis
Descriptive statistics were used to summarize demographic and clinical characteristics of subjects with and without PPC. Categorical variables were summarized as counts and percentages and compared using Chi-Square tests or Fisher’s Exact tests as appropriate. Continuous data were summarized using medians and interquartile ranges (IQR) and compared using the Mann-Whitney U test. All data were analyzed using SAS 9.4 for Windows (SAS Inst. Cary, NC).
RESULTS
Table 1 shows sample demographics and clinical features of the 1389 patients admitted to the CICU compared by PPC consult. More than 100 (n=112; 8%) patients received a consult. Overall, patients who received PPC were younger at time of their first admission compared to patients who did not (63 d vs. 239 d; p =0.003). While patients younger than 30 days comprise the largest age group in both cohorts, there was a greater proportion of these patients in the PPC group (p=0.01). Patients admitted to the CICU with and without PPC were mainly surgical (67% vs. 78%); however, patients treated medically were underrepresented in the non-PPC group (p=0.012).
Table 1.
Sample Demographics and Clinical Features Compared by Pediatric Palliative Care Consult (N=1389).
| All Patients | (+) PPC (n = 112) | (−)PPC (n = 1,277) | P |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 60 (54) | 722 (57) | 0.544 |
| Residency, n (%) | |||
| In –State | 83 (74) | 966 (76) | |
| Religion, n (%) | |||
| LDS | 54 (48) | 591 (46) | |
| Other | 29 (26) | 462 (36) | |
| None | 29 (26) | 224 (18) | 0.029 |
| Age at first hospital admission, median (IQR) | 63d (0d – 1,954) | 239d (3d – 2695d) | 0.003 |
| Age distribution at first hospital admission, n (%) | |||
| < 30 days | 51 (46) | 413 (32) | |
| 1 month-12 months | 15 (13) | 262 (21) | |
| 12 month-5years | 18 (16) | 193 (15) | |
| 5 years to 12 years | 21 (19) | 208 (16) | |
| 12 years to 18 years | 5 (4) | 174 (14) | |
| >18 years | 2 (2) | 27 (2) | 0.010 |
| Treatment of Heart Disease n (%) | |||
| Surgical | 75 (67) | 992 (78) | |
| Cardiac Catheterization | 20 (18) | 185 (14) | |
| Medical | 17 (15) | 100 (8) | 0.012 |
IQR = Interquartile range, PPC= Pediatric Palliative Care, LDS= Church of Jesus Christ of Latter-day Saints, Other= Catholic, Lutheran, Hindu, Buddhist, Christian, Protestant, Baptist, Muslim, Unitarian, Methodist, Jehovah Witness, Pentecostal, Bahai Faith, Greek Orthodox, Assembly of God, Seventh- day Adventist.
Table 2 shows disease severity compared by receipt of PPC consult. Patients with a consult had a higher median number of CCCs at the end of first hospitalization (3 vs. 1; p<0.001) and at the end of study period (4 vs. 2; p <0.001) compared to those who did not. Of patients with a consult, over half (60%) had three or more CCCs at the end of first hospitalization, whereas, two-thirds (82%) without a consult had two or fewer CCCs. The most common CCC in both groups was congenital/genetic defect. In addition, patients in the PPC group had longer cumulative HLOS (60 days vs. 7d; p<0.001), CICU LOS (11d vs. 2d; p=0.001) and a higher mortality rate (38%vs. 3%; p<0.001) compared to the non-PCC group.
Table 2.
Disease Severity by Receipt of Pediatric Palliative Care Consult (N=1389).
| All Patients | (+) PPC (n = 112) | (−) PPC (n=1,277) | P value |
|---|---|---|---|
| Complex Chronic Conditions | |||
| CCCs at the end of first hospitalization, median (IQR) | 3 (2–5) | 1 (1–2) | <0.001 |
| End of Study CCCs, median (IQR) | 4 (3–6) | 2 (1–3) | < 0.001 |
| CCCs at the end of first hospitalization, n (%) | |||
| 0 | 1 (1) | 38 (3) | |
| 1–2 | 44 (39) | 1012 (79) | |
| 3–4 | 37 (33) | 195 (15) | |
| 5–6 | 25 (22) | 28 (2) | |
| ≥7 | 5 (4) | 4 (1) | <0.001 |
| Five most common CCCs categories at the end of first hospital admission, n (%) | |||
| Congenital or genetic defect | 55 (49) | 294 (23) | < 0.001 |
| Gastrointestinal | 50 (45) | 118 (9) | < 0.001 |
| Premature and neonatal | 34 (30) | 182 (14) | < 0.001 |
| Neurologic | 32 (29) | 80 (6) | < 0.001 |
| Renal and urologic | 23 (21) | 56 (4) | < 0.001 |
| Hospital and CICU LOS | |||
| Total CICU days, median (IQR) | 11 (5–30) | 2 (1 – 7) | < 0.001 |
| Total hospital days, median (IQR) | 60 (22–100) | 7 (3–19) | < 0.001 |
| Mortality, n (%) | |||
| Mortality | 42 (38) | 43 (3) | < 0.001 |
IQR = Interquartile range, PPC= Pediatric palliative care, CCC= complex chronic condition, CICU= Cardiac Intensive Care Unit, LOS= length of stay.
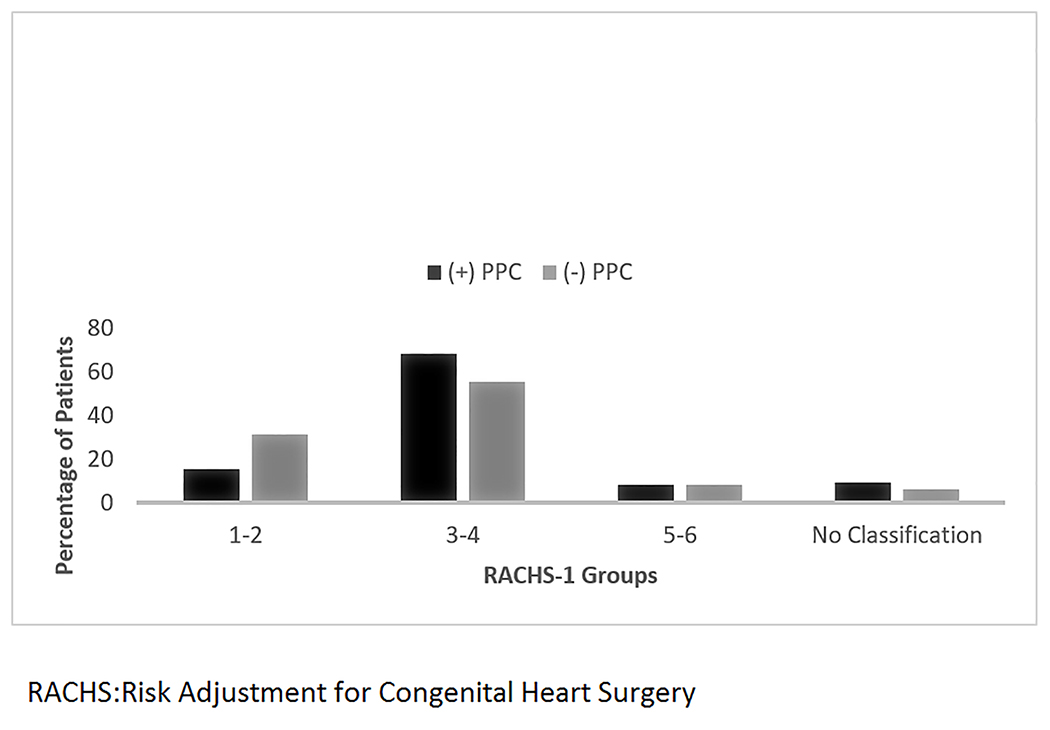
Figure 1 shows the RACHS groups distribution among surgical patients. Patients in the RACHS 3–4 group comprise the largest percentage of patients in both the PPC (68%) and non-PPC group (55%). In addition, this RACHS category group also had the largest number of children under the age of 30 days (not shown in Figure).
Figure 1:
Surgical Patients using Risk Adjustment for Congenital Heart Surgery Classification by Palliative Care Consult
PPC= Pediatric palliative care
Characteristics of patient deaths by PPC consult are displayed in Table 3. The PPC patients were older at time of death (140 d vs. 71 d; p=0.031), had a higher median number of CCCs at the end of first hospitalization, and at the time of death (3 vs. 2; p< 0.001), and a longer cumulative HLOS (58 d vs. 18 d; p <0.001) than the non-PPC group. The location and mode of death varied by receipt of PPC consult; patients with a PPC consult were more likely to have comfort care at the end of life (36% vs.2%; p=0.002) and died at home on hospice (24% vs. 2%; p=0.020) compared to those who did not receive a consult.
Table 3:
Characteristics of Patient Deaths Compared by Pediatric Palliative Care Consult (N=85).
| Patient Deaths | (+)PPC (n = 42) | (−) PPC (n = 43) | P |
|---|---|---|---|
| Age at time of death | |||
| Age in days, median (IQR) | 140 (65–261) | 71 (16–201) | 0.031 |
| Complex Chronic Conditions | |||
| CCCs at the end of first hospitalization, median (IQR) | 3 (2– 4) | 2 (1– 2) | <0.001 |
| CCCs at time of death, median (IQR) | 3 (2–5) | 2 (1– 2) | <0.001 |
| Hospital and CICU LOS | |||
| CICU LOS in days, median (IQR) | 16 (5–52) | 10 (3–22) | 0.055 |
| Hospital LOS in days, median (IQR) | 58 (19–90) | 18 (3–35) | 0.001 |
| Location of Death, n (%) | |||
| Intensive care unit | 28 (67) | 31 (72) | |
| Home on hospice | 10 (24) | 1 (2) | |
| Home unexpected | 1 (2) | 2 (5) | |
| Hospital, not ICU | 2 (5) | 8 (19) | |
| Chronic care facility | 1 (2) | 0 (0) | |
| Unknown | 0 | 1 (2) | 0.020 |
| Modes of death, n (%) | |||
| Withdrawal of life-sustaining therapies | 21 (50) | 28 (65) | |
| Comfort care | 15 (36) | 1 (2) | |
| Died during resuscitation | 5 (12) | 11 (26) | |
| No Escalation of therapy | 1 (2) | 2 (5) | |
| Unknown | 0 | 1 (2) | 0.002 |
IQR = Interquartile range, PPC= Pediatric palliative care, CCC= complex chronic condition, CICU= Cardiac Intensive Care Unit, LOS= length of stay.
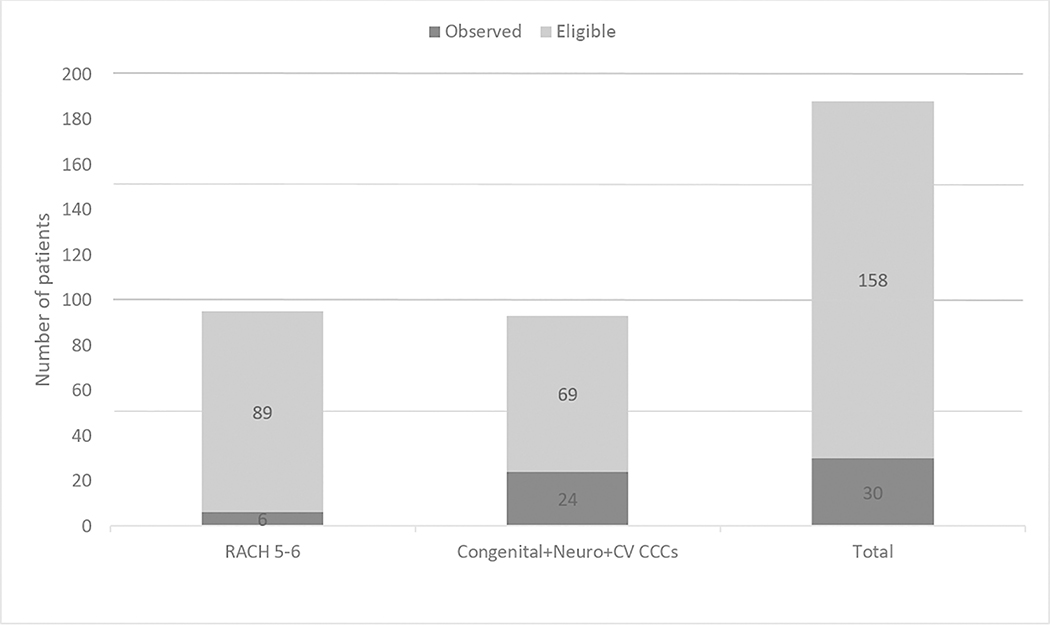
Based on CAPC criteria, we evaluated the rate of PPC consults among two groups; 1) the RACHS 5–6 surgical patients; and 2) patients with heart disease who also had a neurological and congenital/genetic CCCs. Using the CAPC criteria, only 6 (7%) of 89 of eligible RACHS 5–6 group and 24 (35%) of the 69 eligible received a consult (Figure 2).
Figure 2:
Observed vs. Eligible Pediatric Palliative Care Consultation Based on Criteria from the Center to Advance Palliative Care.
Neuro = Neurologic, CV = Cardiovascular, RACHS = Risk Adjustment for Congenital Heart Surgery, CCC= Complex Chronic Condition
DISCUSSION
We compared the characteristics of pediatric patients, with all types of heart disease treated in a dedicated CICU, who received a PPC consult to patients who did not. Our study has three main findings. First, only 8% of patients received a PPC consult with the most complex surgical patients missing a referral when CAPC guidelines were retrospectively applied. Second, PPC patients were younger, had higher disease severity measured by CCCs, longer LOS, and higher mortality compared to patients without a PPC consult. Third, most patients died in the ICU after the withdrawal of life-sustaining therapies regardless of receipt of PPC consult. However, patients with a consult were most likely to receive comfort care at the end of life and die at home.
Less than 10% of patients with heart disease admitted to a dedicated CICU received a PPC consult. While previous studies have evaluated PPC consults for patients with advanced heart disease, defined as severe heart failure symptoms frequently requiring high technology therapies (21), our study is unique in its evaluation of rates of PPC consult in patients with all types of heart disease. Keele and colleagues reported varying but increasing overall rates of PPC utilization from 1 to 8%. However, their study included all patients who died in the hospital with different disease processes. The authors found that receipt of PPC varied by major diagnostic codes, with the highest proportion found among children with neurologic disease and lowest in neonates and children with circulatory diseases (9). Criteria for PPC consult suggested by CAPC are not widely used, and the impact of the criteria have not been reported. Application of the CAPC criteria to our cohort demonstrated low rates of PPC consult; only a few patients within the RACHS 5–6 category and children with combined neurological and congenital/genetic defects received a PPC consult. In contrast, patients with lower RACHS scores were more likely to receive PPC consult as these patients had underlying comorbidities and/or a protracted hospital stay with adverse outcomes. This finding emphasizes that providers may not effectively predict outcomes or the need for PPC, based on surgical complexity alone. Instead, the continuous appraisal of critical illness trajectory and assessment of prognosis should guide decisions for obtaining a PPC consult (22).
We found that patients who received a PPC consult were younger, experienced longer hospitalization, had a higher number of CCCs, and higher mortality rates compared to patients who did not. In addition, newborns and surgical patients predominated in the PPC cohort. The most complex surgical patients with RACHS 5–6, who traditionally experience the highest morbidity and mortality risk (19), had low rates of PPC consult. These findings demonstrate multifactorial barriers toward PPC from the misconception that PPC consults are only useful for end of life issues, to providers’ fear of undermining parental hope and giving the impression of giving up (23,24). The use of clinical and surgical characteristics as criteria for PPC referral in patients with heart disease has the possibility to facilitate earlier and more frequent PPC involvement.
In our study, patients with and without a PPC consult died in the ICU in similar proportions after withdrawal of life-sustaining therapies. Notably, patients with a PPC consult were more likely to receive comfort care at the end of life, less likely to die during resuscitation and more likely to die at home. Trowbridge et al., found that children with PPC consults were less likely to die during resuscitation and more likely to die with no escalation of support (6). Wolfe and colleagues showed that a boosted awareness of caregivers regarding early involvement of PPC resulted in an increase of hospice discussions between caregivers and families, early documentation of Do Not Resuscitate orders, and a decrease in the number of inpatient deaths (25). Families report home as the preferred location of death for their hospitalized child (26). However, rates of pediatric death at home or in a hospice facility have remained less than 40% (27). In-hospital pediatric death is a complicated and challenging phenomenon. As opposed to adult medicine, in-hospital pediatric death is not a poor-quality end of life care outcome (28). Many factors determine in-hospital versus home death in the pediatric population. Factors include the patient’s condition, sense of security in the hospital setting, concerns of symptom control outside the hospital, impact of home death on siblings, adequacy of home palliative care and hospice staffing, and delayed PPC referral among others (26, 29). This leaves many opportunities to improve buy-in by multiple service lines, identify effective ways to implement palliative care services earlier and reduce the burden of heart disease to patients and their families.
The study has limitations. As a single-center study, there are issues with generalizability; our study looked at a broad range of cardiac diseases and did not focus solely on children with advanced heart disease. PPC referral at our institution is not automatic, but rather dependent on a licensed health care provider’s independent decision. Other centers may have different referral mechanisms, making comparisons difficult. Moreover, we do not know if there were families who refused a PPC consultation. This may have resulted in under reporting of PPC consults in our study. Additionally, the timing of the PPC consult is unknown in relation to the diagnosis of heart disease. Lastly, we collected data from an administrative database, and there may be under or over-reporting of CCCs related to possible misclassifications in ICD-10 coding.
CONCLUSION
This study highlights PPC utilization in patients with heart disease treated in a dedicated ICU and increases awareness about PPC within our institution and globally. We found that PPC consults occurred infrequently in the context of high disease burden, complexity, and lower survival. Importantly, children who had a PPC consult were more likely to receive comfort care at the end of life and die at home. It appears from retrospectively applying the CAPC criteria that PPC should have been utilized more often.
Future studies are warranted to understand the facilitators and barriers to a health care provider making a PPC referral. It will be important to evaluate how a referral criteria will help connect families with PPC in a timely manner and to measure the qualitative impact of PPC consultation on families and patients with heart disease.
ACKNOWLEDGEMENTS
The authors wish to acknowledge Kelly Mansfield RN, and Randall Smout, MS, for their help with data collection.
Author Disclosure Statement
Sarah Wawrzynski, RN is predoctoral fellow supported by National Institute of Nursing Research of the National Institutes of Health under award number T32NR013456 (m-PIs: Ellington and Mooney)
Footnotes
All other authors have no financial or potential conflicts of interest to disclose.
Copyright form disclosure: Dr. Wawrzynski’s institution received funding from National Institute of Nursing Research; she received support for article research from the National Institutes of Health; and she disclosed government work. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Friedrichsdorf SJ, Postier A, Dreyfus J, et al. Improved quality of life at end of life related to home-based palliative care in children with cancer. J Palliat Med. 2015;18:143–150 [DOI] [PubMed] [Google Scholar]
- 2.Sheetz MJ, Bowman M-AS. Parents’ perceptions of a pediatric palliative program. Am J Hosp Plliat Care. 2013;30:291–296 [DOI] [PubMed] [Google Scholar]
- 3.Mazwi ML, Kirsch R. The role of palliative care in critical congenital heart disease. Semin Perinatol. 2017;41:128–132 [DOI] [PubMed] [Google Scholar]
- 4.May R, Thompson J. The role of pediatric palliative care in complex congenital heart disease: Three Illustrative Cases. J Palliat Med. 2017;20:1300–1303 [DOI] [PubMed] [Google Scholar]
- 5.Ullrich CK, Lehmann L, London WB, et al. End-of-life care patterns associated with pediatric palliative care among children who underwent hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2016;22:1049–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trowbridge A, Walter JK, McConathey E, et al. Modes of death within a children’s hospital. Pediatrics. 2018;142:e20174182. [DOI] [PubMed] [Google Scholar]
- 7.Goldhagen J, Fafard M, Komatz K, et al. Community-based pediatric palliative care for health related quality of life, hospital utilization and costs lessons learned from a pilot study. BMC Palliat Care. 2016;15(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pierucci RL, Kirby RS, Leuthner SR, et al. End-of-life care for neonates and infants: The experience and effects of a palliative care consultation service. Pediatrics. 2001;108:653–660 [DOI] [PubMed] [Google Scholar]
- 9.Keele L, Keenan HT, Sheetz J, et al. Differences in characteristics of dying children who receive and do not receive palliative care. Pediatrics. 2013;132:72–78 [DOI] [PubMed] [Google Scholar]
- 10.Osenga K, Postier A, Dreyfus J, et al. A comparison of circumstances at the end of life in a hospital setting for children with palliative care involvement versus those without. 2016. J Pain Symptom Manage. 2016; 52:673–680 [DOI] [PubMed] [Google Scholar]
- 11.Mosenthal AC, Weissman DE, Curtis JR, et al. Integrating palliative care in the surgical and trauma intensive care unit: a report from the Improving Palliative Care in the Intensive Care Unit (IPAL-ICU) project Advisory Board and the Center to Advance Palliative Care. Crit Care Med. 2012;40:1199–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Academy of Pediatrics Policy Statement: Pediatric palliative care and hospice care commitments, guidelines, and recommendations. Pediatrics. 2013;132;966–972 [DOI] [PubMed] [Google Scholar]
- 13.Boss R, Nelson J, Weissman D, et al. Integrating palliative care into the PICU. Pediatr Crit Care Med. 2014;15:762–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alsoufi B The effect of noncardiac and genetic abnormalities on outcomes following neonatal congenital heart surgery. Semin Thorac Cardiovasc Surg. 2016;28:114–117 [DOI] [PubMed] [Google Scholar]
- 15.McCracken C, Spector LG, Menk JS, et al. Mortality following pediatric congenital heart surgery: An analysis of the causes of death derived from the national death index. J Am Heart Assoc. 2018;7:e010624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Latal B, Helfricht S, Fischer JE, et al. Psychological adjustment and quality of life in children and adolescents following open-heart surgery for congenital heart disease: a systematic review. BMC Pediatr. 2009;9:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friebert S, Osenga K. Center to Advance Palliative Care. Pediatric palliative care referral criteria. http://www.capc.org/old-tools-for-palliative-careprograms/clinical-tools/consult-triggers/pediatric-palliative-care-referral-criteria. Published 2009 Accessed March 4, 2019
- 18.Feudtner C, Feinstein JA, Zhong W, et al. Pediatric complex chronic conditions classification system version 2: Updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenkins KJ. Risk adjustment for congenital heart surgery: The RACHS-1 method. Pediatr Card Surg Annu. 2004;7:180–184 [DOI] [PubMed] [Google Scholar]
- 20.Blinderman CD, Billings A. Comfort care for patients dying in the hospital. N Engl J Med. 2015;373: 2549–2561 [DOI] [PubMed] [Google Scholar]
- 21.Marcus KL, Balkin EM, Al-Sayegh H, et al. Patterns and outcomes of care in children with advanced heart disease receiving palliative care consultation. J Pain Symptom Manage. 2018;55:351–358 [DOI] [PubMed] [Google Scholar]
- 22.Gilleland JC, Parshuram CS. Discussing death as a possible outcome of PICU Care. Pediatr Crit Care Med. 2018;19:S4–S9 [DOI] [PubMed] [Google Scholar]
- 23.Balkin EM, Sleeper LA, Kirkpatrick JN, et al. Physician perspectives on palliative care for children with advanced heart disease: A comparison between pediatric cardiology and palliative care physicians. J Palliat Med. 2018;21:773–779 [DOI] [PubMed] [Google Scholar]
- 24.Balkin EM, Kirkpatrick JN, Kaufman B, et al. Pediatric cardiology provider attitudes about palliative care: A multicenter survey study. Pediatr Cardiol. 2017; 38:1324–1331 [DOI] [PubMed] [Google Scholar]
- 25.Wolfe J, Hammel JF, Edwards KE, et al. Easing of suffering in children with cancer at the end of life: is care changing? J Clin Oncol. 2008;26:1717–1723 [DOI] [PubMed] [Google Scholar]
- 26.Kassam A, Skiadaresis J, Alexander S, et al. Parent and clinician preferences for location of end-of-life care: home, hospital or freestanding hospice? Pediatr Blood Cancer. 2014;61:859–864 [DOI] [PubMed] [Google Scholar]
- 27.Friebert S, Williams C. National Hospice and Palliative Care Organization’s Facts and Figures. 2015. Available from: https://www.gippcc.org/wp-content/uploads/2017/06/pediatric-facts-figures.pdf. Accessed March 4, 2019.
- 28.Johnson EE, Rosenberg AR, Kamil AH. Pediatric-specific end of life care quality measures: An unmet need of a vulnerable population. J Oncol Pract. 2017. 13:e874–e880 [DOI] [PubMed] [Google Scholar]
- 29.Dussel V, Kreicbergs U, Hilden JM, et al. Looking beyond where children die: determinants and effects of planning a child’s location of death. J Pain Symptom Manage. 2009;37:33–43 [DOI] [PMC free article] [PubMed] [Google Scholar]