Figure 2. The reaction chamber and oleoyl-CoA binding site.
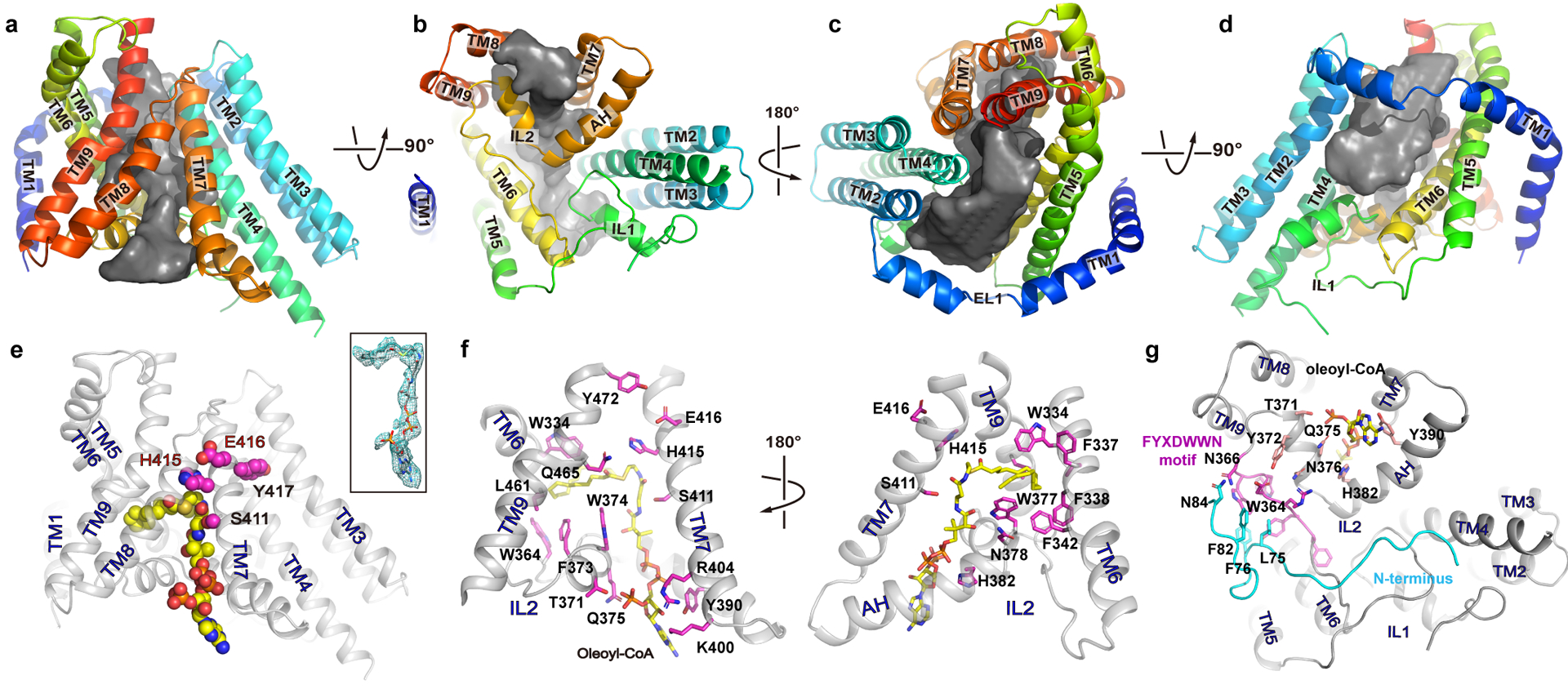
a-d. The reaction chamber (grey surface) is shown in four orientations with the surrounding helices as cartoon. e. an oleoyl-CoA is shown as spheres with carbon atoms colored in yellow. The side chains of the conserved active site residues (SXXHEY) are shown as magenta spheres. inset: oleoyl-CoA in stick and its density as green mesh. f. Residues at the oleoyl-CoA binding site are shown as sticks with carbon atoms colored in magenta. g. Interaction between the FYXDWWN motif (magenta) and the N-terminus of the neighboring protomer (cyan).
