Extended Data Figure 1. Side-by-side comparison of hDGAT1 and DltB.
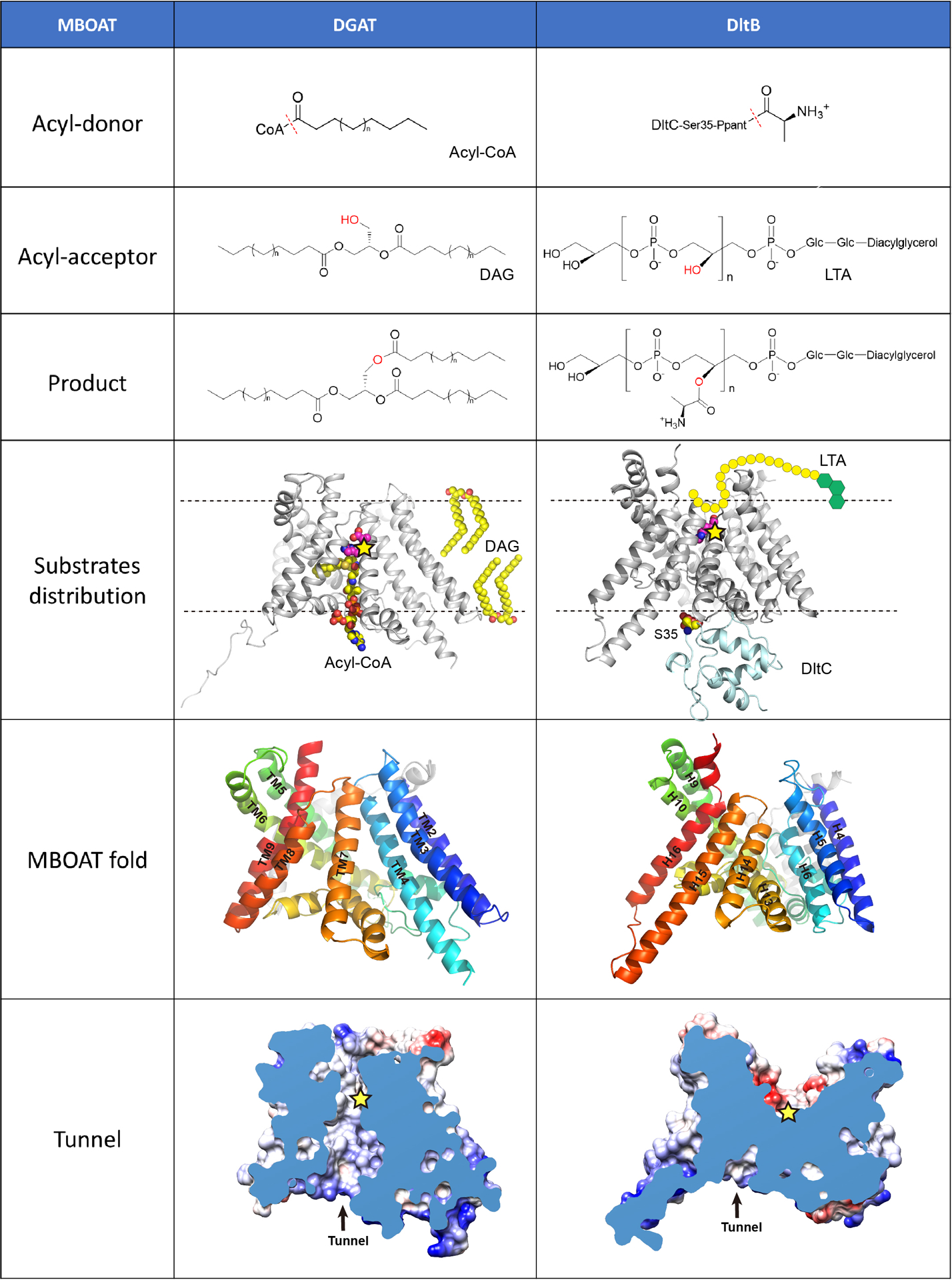
Both hDGAT1 and DltB have an acyl-donor and an acyl-acceptor. In the “acyl-donor” row, the red dashed lines indicate the bonds that are broken during acyl-transfer reactions. In the “acyl-acceptor” row, the hydroxyl groups are highlighted in red. In the “substrate distribution” row, hDGAT1 and DltB-DltC complex are shown as cartoon and the membrane as dashed lines. The position of the catalytic histidine in each protein is marked as a yellow star. In hDGAT1, acyl-CoA comes from the intracellular side while DAG comes from the hydrophobic core of membrane. In DltB, the Ppant-DltC is intracellular while the lipoteichoic acid (LTA) is extracellular. In the “MBOAT fold” row, the MBOAT folds of hDGAT1 and DltB are shown in cartoon representations and viewed from the same orientation. Equivalent helices have the same color. The “tunnel” row shows the cut-away surface illustrations of hDGAT1 and DltB featuring their cytosolic tunnels. The position of the conserved histidine residue is marked as a yellow star. In DltB, the intracellular loops are placed more towards the center of the membrane and as a result, the MBOAT fold in DltB does not carve out a reaction chamber in the membrane. Overall, DltB is shaped like an hourglass that allows the two substrates to approach the reaction center from either sides of the membrane, and the transfer of an acyl group across the membrane. These observations highlight the versatility of the MBOAT fold.
