Fig. 1. Plasma viral loads, CD4+ T-cell count and HAART regimens at different time points over a 14-year period for the patient.
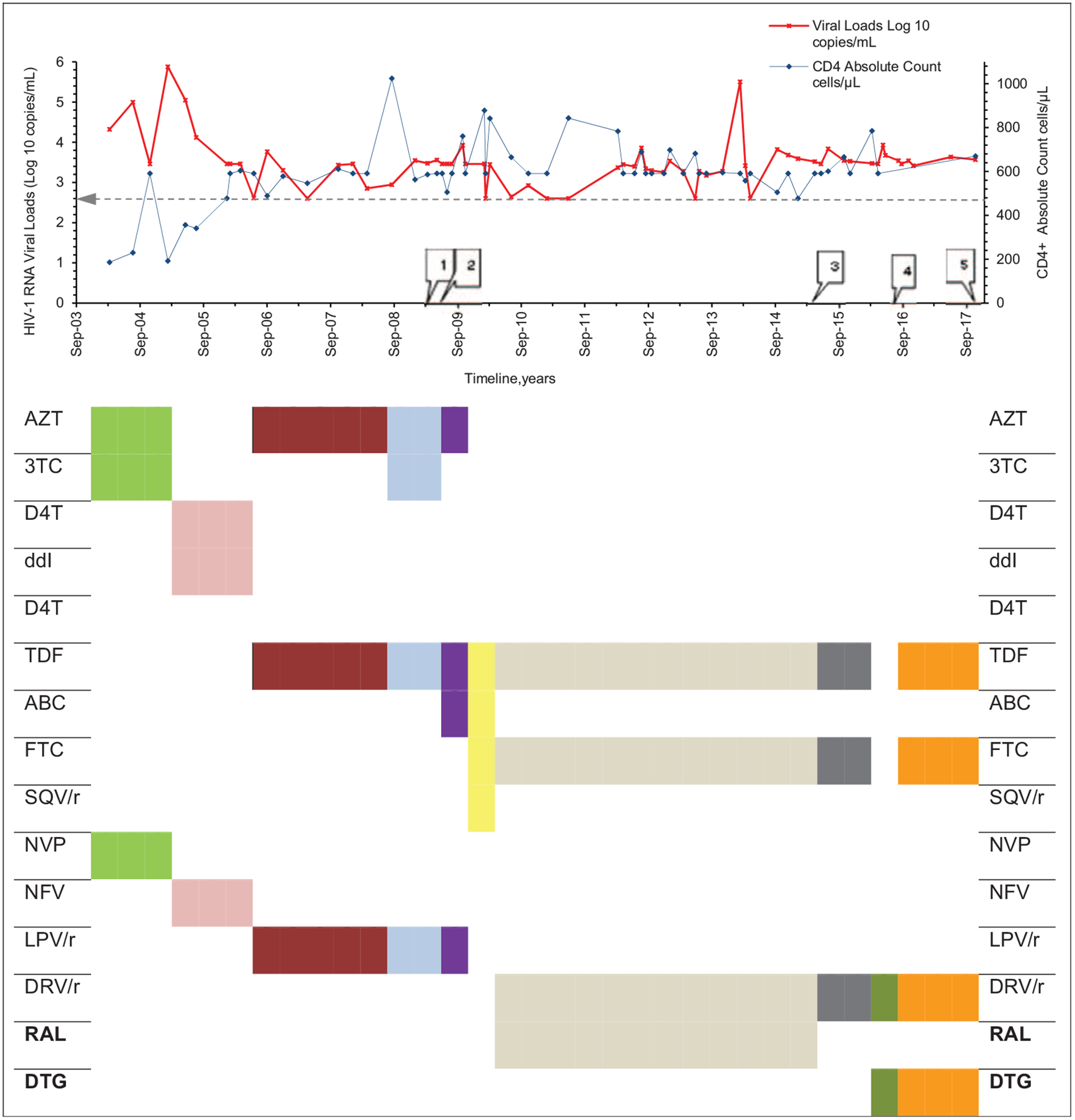
Horizontal line within chart depicts viral load of 400 copies/ml. Numbered rectangular callouts depict when drug resistance testing was done. (1) Mar 2009; †nucleotide reverse-transcriptase inhibitors, D67N, K70R, M184V; nonnucleotide reverse-transcriptase inhibitors, Y181C; protease inhibitors major resistance mutations, V32I, I47V, I54L, I84V, †protease inhibitors accessory resistancemutations, L33F, G73V, L89T; integrase strand transfer inhibitors, not tested. (2) Apr 2009; †nucleotide reverse-transcriptase inhibitors, D67N, K70R, M184V, K219N; †nonnucleotide reverse-transcriptase inhibitors, Y181C; †protease inhibitors major resistance mutations, V32I, I47V, I54L, I84V, protease inhibitors accessory resistance mutations, L33F, G73V, L89T; integrase strand transfer inhibitors, not tested. (3) May 2015; †nucleotide reverse-transcriptase inhibitors, K65KR, D67DN, K70KR, M184MV, K219KHNQ; †nonnucleotide reverse-transcriptase inhibitors, none; †protease inhibitors major resistance mutations, V32VI, I54IL, I84IV, †protease inhibitors accessory resistance mutations, L33LF, G73GV, L89IMT; †integrase strand transfer inhibitors major resistance mutations, E138K, G140A, Q148R, integrase strand transfer inhibitors accessory resistance mutations, none; (4) Aug 2016; †nucleotide reverse-transcriptase inhibitors, D67N, K70R, M184V; †nonnucleotide reverse-transcriptase inhibitors, none; †protease inhibitors major resistance mutations, V32I, I47V, I54L, I84V, †protease inhibitors accessory resistance mutations, L33F, G73V, L89T; integrase strand transfer inhibitors, not tested. (5) Nov 2017; †integrase strand transfer inhibitors major resistance mutations, E138K, G140A, S147G, Q148R, integrase strand transfer inhibitors accessory resistance mutations, T97A; ‡fusion inhibitors, none; entry inhibitors, §R5 tropic; nucleotide reverse-transcriptase inhibitors, not tested; nonnucleotide reverse-transcriptase inhibitors, not tested. 3TC, lamivudine; ABC, abacavir; AZT, zidovudine; d4T, didanosine; ddI, didanosine; DRV/r, ritonivir boosted darunavir; DTG, dolutegravir; EI, entry inhibitor; FI, fusion inhibitor; FTC, emtricitabine; INSTI, integrase strand transfer inhibitor; LPV/r, ritonovir boosted lopinavir; NFV, nelfinavir; NNRTI, nonnucleotide reverse-transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; NVP, nevirapine; PI, protease inhibitor; RAL, raltegravir; RM, resistance mutation; SQV/r, ritonivir-boosted saquinavir; TDF, tenofovir; VF, virological failure. Drug resistance mutations interpreted using †Stanford University HIV Drug resistance database; ‡International Antiviral Society USA 2017 mutational list; §coreceptor usage assessed with geno2pheno[coreceptor]. Source: modified version from http://bioafrica.mrc.ac.za/workshops/PDFs/deOliveiraRegaDB.pdf.
