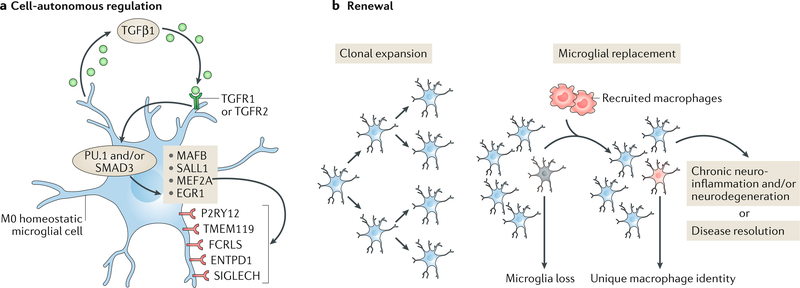
Fig. 1 |. Regulation of microglia homeostasis.
a | Microglia are maintained in a cell-autonomous fashion via transforming growth factor-β1 (TGFβ1) signalling, which drives the transcriptional regulation (via the transcription factors PU.1 and/or mothers against decapentaplegic homologue 3 (SMAD3)) of genes that include those encoding the transcription factor MafB (MAFB), Sal-like protein 1 (SALL1), myocyte-specific enhancer factor 2A (MEF2A) and early growth response protein 1 (EGR1)25,75. Several unique surface receptors — P2Y purinoceptor 12 (P2RY12), transmembrane protein 119 (TMEM119), Fc receptor-like S, scavenger receptor (FCRLS), ectonucleoside triphosphate diphosphohydrolase 1 (ENTPD1; also known as CD39) and sialic acid binding Ig-like lectin H, isoform CRA_a (SIGLECH) — identify microglia. P2RY12 senses damaged tissue through the detection of extracellular nucleotides53, whereas ENTPD1 controls microglial process ramification218 and its loss is associated with handling-induced seizures in mice219. The role of the other receptors is as yet unknown. b | Microglial renewal occurs by local clonal expansion13. Furthermore, under certain conditions, lost microglial populations can be replaced with peripherally derived macrophages that are recruited to the CNS. These cells maintain a unique molecular identity and have distinct functional roles as compared with microglia17, although TMEM119 and other microglial markers may also be expressed by these macrophages220. Loss of TGFβ1 signalling in these macrophages has been shown to lead to a progressive and fatal demyelinating motor disease18, suggesting that the manipulation of engrafted myeloid cells has therapeutic potential in neurodegenerative disease. TGFR1 and TGFR2, TGFβ receptors type 1 and 2, respectively.