Objectives:
To investigate patients’ characteristics, management, and outcomes in the critically ill population admitted to the ICU for severe acute respiratory syndrome coronavirus disease 2019 pneumonia causing an acute respiratory distress syndrome.
Design:
Retrospective case-control study.
Setting:
A 34-bed ICU of a tertiary hospital.
Patients:
The first 44 coronavirus disease 2019 acute respiratory distress syndrome patients were compared with a historical control group of 39 consecutive acute respiratory distress syndrome patients admitted to the ICU just before the coronavirus disease 2019 crisis.
Interventions:
None.
Measurements and Main Results:
Obesity was the most frequent comorbidity exhibited by coronavirus disease 2019 patients (n = 32, 73% vs n = 11, 28% in controls; p < 0.001). Despite the same severity of illness and level of hypoxemia at admission, coronavirus disease 2019 patients failed more high flow oxygen via nasal cannula challenges (n = 16, 100% vs n = 5, 45% in controls; p = 0.002), were more often intubated (n = 44, 100% vs n = 22, 56% in controls; p < 0.001) and paralyzed (n = 34, 77% vs n = 3, 14% in controls; p < 0.001), required higher level of positive end-expiratory pressure (15 vs 8 cm H2O in controls; p < 0.001), more prone positioning (n = 33, 75% vs n = 6, 27% in controls; p < 0.001), more dialysis (n = 16, 36% vs n = 3, 8% in controls; p = 0.003), more hemodynamic support by vasopressors (n = 36, 82% vs n = 22, 56% in controls; p = 0.001), and had more often a prolonged weaning from mechanical ventilation (n = 28, 64% vs n = 10, 26% in controls; p < 0.01) resulting in a more frequent resort to tracheostomy (n = 18, 40.9% vs n = 2, 9% in controls; p = 0.01). However, an intensive management requiring more staff per patient for positioning coronavirus disease 2019 subjects (6 [5–7] vs 5 [4–5] in controls; p < 0.001) yielded the same ICU survival rate in the two groups (n = 34, 77% vs n = 29, 74% in controls; p = 0.23).
Conclusions:
In its most severe form, coronavirus disease 2019 pneumonia striked preferentially the vulnerable obese population, evolved toward a multiple organ failure, required prolonged mechanical ventilatory support, and resulted in a high workload for the caregivers.
Keywords: acute respiratory distress syndrome, coronavirus, coronavirus disease 2019, mechanical ventilation, obesity
The world has recently been facing a rapidly spreading infectious epidemic, first appearing in China in December 2019, due to a new coronavirus called the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The high contagiousness of this coronavirus disease 2019 (COVID-19) caused a massive influx of patients to hospitals with severe forms of lower respiratory tract infections. Most ICUs in Europe and United States have now been overwhelmed by a surge of critically ill patients exhibiting a life-threatening form of COVID-19 induced acute respiratory distress syndrome (COVID-19 ARDS) (1). Most of the published data currently available originated from the first Chinese cluster of the epidemic. In a Chinese population of 1590 patients, Guan et al (2) showed that patients’ comorbidities worsen the prognosis with a higher chance to need mechanical ventilation and to die in those having two or more comorbidities compared with healthier groups. However, only 8.2% of their population reported having two or more comorbidities, which may not mirror the critically ill population commonly seen in Europe or the United States, in particular with regards to prevalence of obesity (3–6). Here, we report on our experience from our first 44 cases of COVID-19 critically ill patients and analyze the link between obesity and this new form of ARDS caused by the SARS-CoV-2 with its potential implications for patients’ management.
MATERIALS AND METHODS
This retrospective observational single-center study was declared to the Commission Nationale de l’Informatique et des Libertés, the national commission of computer science and liberty. As the data were collected in an anonymized protected electronic file, ethical review was waived.
Inclusion Criteria
We included the first consecutive 44 cases of COVID-19 ARDS admitted to our adult ICU. They were compared with a historical control group made of the last 39 cases of non-COVID-19 ARDS admitted in the 5 months period preceding this COVID-19 crisis. Because of the rapidly growing number of critically ill candidates for the ICU, our hospital increased its capacity from 10 to 34 ICU beds, all dedicated to COVID-19 ARDS patients. The diagnosis of ARDS was based on the Berlin definition of the syndrome (7). The underlying cause of ARDS was identified after a thorough medical investigation, combining clinical examination, laboratory tests, multiple samples for viral and bacterial analyses, and imaging with chest radiograph and/or chest CT scan. The diagnosis of COVID-19 ARDS was confirmed when a patient met the ARDS criteria and had either a positive COVID-19 virus test by real-time polymerase chain reaction on an upper and/or lower respiratory tract sample or a typical clinical presentation associated with characteristic imaging features on CT scan (8, 9). The latter include bilateral septal lines and diffuse ground-glass opacities predominantly located in the subpleural spaces, consolidations, and the vacuole sign (9, 10). Obesity was defined according to the World Health Organization (WHO) classification (11) as a body mass index (BMI) exceeding 30 kg/m2, while stage 2 (severe) obesity referring to BMI greater than 35 kg/m2 and stage 3 (morbid) obesity corresponding to BMI greater than 40 kg/m2.
Patients’ Management
According to our local guidelines and experts recommendations (8, 12), patients admitted in the ICU for severe type 1 (hypoxemic) acute respiratory failure were initially managed using high flow oxygen via nasal cannula (HFO2NC) with the highest flow tolerated (usually at 50 L/min) and the minimal Fio2 required to achieve arterial oxygen saturation (Sao2) greater than 92%. Given the high risk of thromboembolic complications described in the COVID-19 disease (13), all the patients of the COVID-19 group were treated by heparin anticoagulation at therapeutic doses, after the first blood sample was taken, and for the entire duration of the ICU stay. Severe cases with respiratory distress and Pao2/Fio2 less than 150 and Fio2 greater than 60% were considered early for intubation since COVID-19 ARDS patients are known to worsen very quickly (8). Once intubated, patients were deeply sedated, and connected to a double branch circuit ICU ventilator on a flow delivered assist control mode with a low tidal volume (6 mL/kgideal body weight) protective mechanical ventilation strategy (12). Those with persistent severe hypoxemia (Pao2/Fio2 < 150) after a recruitment maneuver were paralyzed (14) and put in prone position for at least 16 hours (15). External positive end-expiratory pressure (PEEP) was set according to a decremental PEEP trial after a recruitment maneuver, to obtain the best compromise between oxygenation (as assessed by the best Sao2 obtained at a certain level of Fio2), respiratory mechanics (the lowest driving pressure as possible), and hemodynamics (the best mean arterial pressure or cardiac output) as proposed in morbidly obese patients with non-COVID-19 ARDS (16).
As recommended, neuromuscular blocking agents (NMBAs) were stopped and sedation was withdrawn as soon as possible. A protocol-driven weaning strategy based on spontaneous breathing trial on T-tube for 30 minutes was systematically used to shorten the weaning process from mechanical ventilation. Obese patients received positive pressure noninvasive ventilation (NIV) immediately after extubation. A tracheostomy was considered in those unweanable from invasive mechanical ventilation beyond the 7th day after intubation, especially when sedation could not be discontinued because of major discomfort, anxiety, and dyspnea promoted by the endotracheal tube. Prolonged weaning referred to an impossible discontinuation of invasive mechanical ventilation 7 days after the first separation attempt from the ventilator (17).
Data Collection
We recorded anthropometric data, cause of ARDS, Charlson Comorbidity Index (18), Clinical Frailty Scale (19), severity at admission as assessed by the Simplified Acute Physiology Score (SAPS) II (20), and the Sepsis-related Organ Failure Assessment score (21), ventilator settings and measurements including plateau pressure (Pplat), total PEEP, driving pressure (difference between Pplat and total PEEP). We also collected the resort to prone position, tracheostomy, and the use of vasopressors, NMBAs, renal replacement therapy. The number of prone position sessions per patient and the number of staff per patient needed to perform prone positioning were also included in the analyses.
Statistical Analysis
The normality of data distribution was assessed using the Shapiro-Wilk test and by visually checking the distribution (histogram) of each variable. Data were expressed as mean ± sd when they were normally distributed and as median and interquartile range (25–75%) when they were non-normally distributed. Proportions were used as descriptive statistics for categorical variables. Comparisons of values between groups were performed using a two-tailed Student t test or Mann-Whitney U test, as appropriate. Pairwise comparisons between admission and prone position were assessed using a paired Student t test or Wilcoxon test, as appropriate. Analyses of discrete data were performed using the chi-square test or Fisher exact test when the numbers were small.
Statistical analyses were performed using IBM SPSS Statistics for Windows (Version 20.0; IBM Corp., Armonk, NY). p value of less than 0.05 was considered statistically significant. All reported p values were two-sided.
RESULTS
The main clinical characteristics of the patients at admission are shown in Table 1. Laboratory data at admission in the ICU are exposed in Table 2. Despite a high prevalence of obesity in our non-COVID-19 control group, we found a significantly higher proportion of obese patients in the COVID-19 ARDS population compared with controls (n = 32, 72% vs n = 11, 28%; p < 0.001). Sixteen percent of the COVID-19 patients exhibited severe obesity (BMI > 35 kg/m2) and the same number of patients (n = 7, 16%) had morbid obesity (BMI > 40 kg/m2).
TABLE 1.
Patients Characteristics at Admission
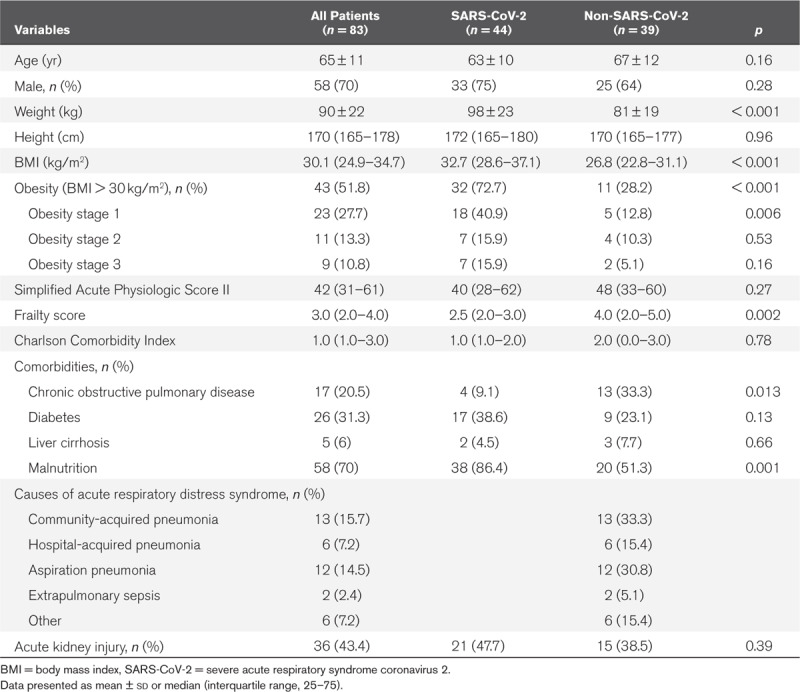
TABLE 2.
Laboratory Data at Admission
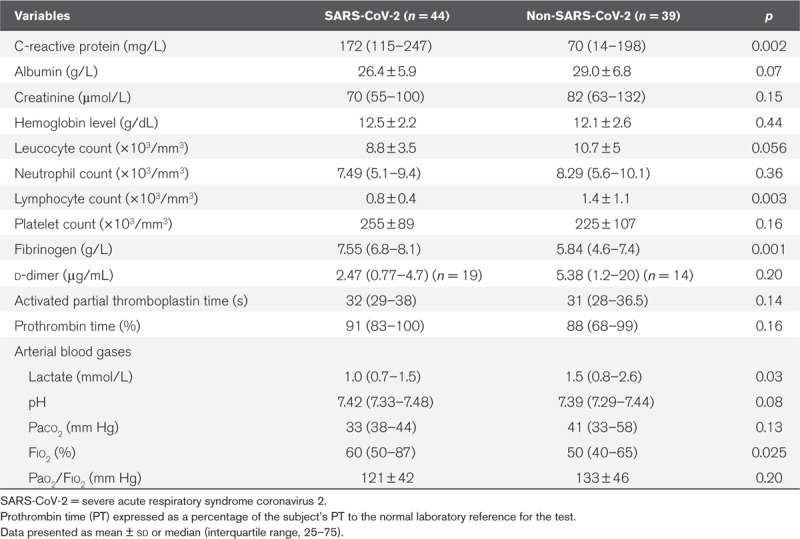
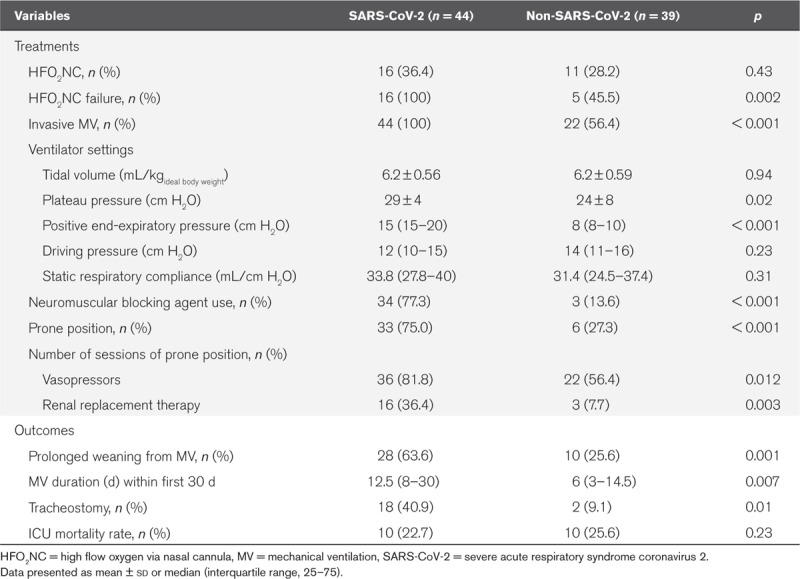
Although the severity of illness according to SAPS II score (40 vs 48 in controls; p = 0.27) and Pao2/Fio2 ratio (121 vs 133 mm Hg in controls; p = 0.20) were similar at admission, COVID-19 patients were intubated more often than controls (n = 44, 100% vs n = 22, 56% in controls; p < 0.001). HFO2NC was attempted in the same proportion of patients in the two groups (n = 16, 36% vs n = 11, 28%; p = 0.43) but it failed more frequently to improve gas exchange in COVID-19 patients (n = 16, 100% vs n = 5, 45% in controls; p < 0.001). COVID-19 patients were more rapidly intubated after an HFO2NC challenge than controls (1.5 d [1.0–2.0 d] vs 5.0 d [3.0–9.0 d]; p < 0.001). As shown in Table 3, the COVID-19 patients were more often paralyzed (n = 34, 77% vs n = 3, 14% in controls; p < 0.001) and put in prone position (n = 33, 75% vs n = 6, 27% in controls; p < 0.001), needed higher PEEP levels (15 vs 8 cm H2O in controls; p < 0.001) compared with controls. After 16 hours of prone positioning, their oxygenation drastically improved (Pao2/Fio2, 113 ± 36 vs 275 ± 84; p < 0.001) but the duration of mechanical ventilation was significantly higher than in the non-COVID-19 patients within the first 30 days of ICU stay (12.5 vs 6 d in controls; p = 0.007). Despite a similar number of patients in acute kidney injury at admission (n = 21, 48% vs n = 15, 39%; p = 0.39), more COVID-19 patients needed renal replacement therapy (n = 16, 36% vs n = 3, 8% in controls; p = 0.003) during their ICU stay. They also required more often an hemodynamic support by vasopressors (n = 36, 81% vs n = 22, 56% in controls; p = 0.012), and more were tracheostomized because of difficult weaning from mechanical ventilation (n = 18, 40.9% vs n = 2, 9%; p = 0.01), but eventually the 28-day mortality was similar in the two groups (n = 10, 22.7% vs n = 10, 25.6%; p = 0.57). Given the higher prevalence of severely obese individuals in the COVID-19 group, these patients required significantly more staff for positioning than the non-COVID-19 ARDS patients (6 [5–7] vs 5 [4–5] in controls; p < 0.001).
TABLE 3.
Patients Management and Outcomes
DISCUSSION
Our study is the first investigation that demonstrates a link between a high prevalence of obesity among critically ill patients admitted for COVID-19 ARDS and a particularly severe clinical course of the disease with challenging situations for the ICU staff. This form of life-threatening COVID-19 pneumonia frequently caused multiple organ failure, was associated with a worst outcome than the usual non-COVID-19 ARDS, and required a higher staffing level.
Several factors may explain why obese patients are more likely to develop a severe presentation of COVID-19-induced pneumonia. First of all, obese patients are especially at risk of more severe respiratory disease than lean subjects due to the pathophysiological consequences of obesity on the respiratory system and immunity (22). Obesity both alters pulmonary gas exchange and respiratory mechanics, especially in the supine position where the abdomen exerts an external compression on the thorax, resulting in an upward shift of the diaphragm (Fig. 1). MacIntyre (23) described this phenomenon with the analogy of a bag-in-box respiratory system where the lungs (the bag) are trapped in a less distensible elastic structure (the chest wall and the abdomen), the box, that hinders the lungs in their expansion capacity. This causes expiratory flow limitation (24, 25) with extensive airway collapse in the dependent lung areas and creates gravitational atelectasis (26), a well-known source of ventilation-perfusion mismatch and severe hypoxemia (27). Interestingly, COVID-19 pneumonia has been described by Gattinoni et al (28) as an atypical ARDS with an apparent discrepancy between a preserved respiratory mechanics with good lung compliance contrasting with a severe alteration of the gas exchanger function of the lungs. The combination of the two factors—COVID-19 pneumonia and obesity—generates a life-threatening complex respiratory picture, challenging to manage. Another explanation for the susceptibility of obese patients to develop severe COVID-19 pneumonia is their impaired ability to respond to infectious agents, especially to viral pathogens. They exhibit a pro-inflammatory state at baseline and a delayed or blunted immune response, resulting in a higher spread of the virus with more damage to the lungs (29).
Figure 1.
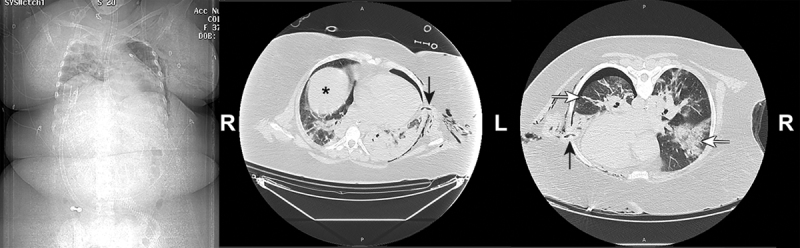
Scout view (on the left) and two slices of chest CT scan acquired in the supine (middle) and prone positions (right) in a 37 yr old massively obese woman (149 kg/158 cm) after 7 d of mechanical ventilation for a severe coronavirus disease 2019 (COVID-19) acute respiratory distress syndrome. The images of the chest tube entering the pleural space (vertical arrows) ensure that the two slices are taken at the same level. Note the upward shift of the right hemidiaphragm (black asterisk) and the gravitational atelectasis of the left lower lobe in supine position. In prone position, the recruitment of the lung is impressive, revealing the pulmonary nodular infiltrates and fibrotic streaks (the horizontal arrows) of COVID-19 pneumonia.
Our findings may have important implications for the management of COVID-19 ARDS patients. Indeed, managing severely obese individuals in ARDS should integrate some specific aspects. Given the potential of obese patients for extremely rapid desaturation (30), the resort to intubation should not be delayed and this intervention must be performed by highly trained intensivists or anesthesiologists using adequate preoxygenation with positive pressure NIV or HFO2NC (31, 32). Adequate protection of the caregivers during preoxygenation and intubation is paramount and includes negative pressure atmosphere and fitted respirator masks (8). A second implication would be to pay a special attention to correct positioning of the critically ill obese patient with COVID-19 ARDS. The ramp position or Head Elevated Laryngoscopy Position facilitates laryngoscopy while it improves pulmonary gas exchange at the same time (33). During mechanical ventilation of the obese subject, priority should be given to prone positioning (15, 34, 35) and sitting position (25) to counteract gravitational atelectasis and lung derecruitment (Fig. 1). For the same reasons, recruitment maneuvers and high PEEP settings are required to maintain a positive transpulmonary pressure and to prevent expiratory lung collapse (16), especially when a protective low tidal volume ventilation is applied (11). De Jong et al (34) have demonstrated the efficacy and feasibility of prone positioning in critically ill massively obese subjects providing that a higher number of caregivers (at least five per patient) is available. Early mobilization of the obese patient and transfer to a chair are an integral part of the weaning process from mechanical ventilation. In the obese patient, NIV is useful immediately after the endotracheal tube has been removed to prevent postextubation respiratory failure, alveolar hypoventilation, and obstructive sleep apnea syndrome (36). Special considerations need to be given to specific bariatric equipment and higher staffing levels, which are challenging in such dramatic epidemic situation and make obesity an additional source of stress for caregivers and healthcare systems (37). The skills and courage of the nursing team are severely tested in these situations at the bedside and should be commended during this worldwide COVID-19 crisis.
Some limitations have to be acknowledged. Given the single-center study design, our results may be considered difficult to extrapolate to another population with a lower prevalence of obesity. However, obesity is a constantly growing epidemic worldwide with about 650 million obese individuals according to the WHO (9). The U.K. Intensive Care National Audit and Research Center reported on COVID-19 in critical care in the United Kingdom and also mentioned a high prevalence (38%) of obesity among 5,578 critically ill patients (5). The Centers for Disease Control and Prevention has also made the same observation in the United States with 48% of COVID-19 patients living with obesity (4). Our cohort may be considered as a small ARDS population, but the literature is seldom focused on a single relatively homogenous cause of ARDS. The COVID-19 crisis gave us the rare opportunity to study a specific form of ARDS caused by a single pathogen. Several methods can be used to set PEEP in ARDS patients in order to reopen the collapsed airways and to improve gas exchange and respiratory mechanics (38). We attempted to standardize our protective mechanical ventilation management of these critically ill obese patients by applying the same practical protocol-driven approach to all the COVID-19 ARDS patients. Because of the massive influx of obese patients, all our residents were trained at the bedside to recruit a critically ill patient under mechanical ventilation according to the method by Pirrone et al (16).
In our experience, COVID-19 pneumonia appeared to strike preferentially the vulnerable obese population and the current collision of two worldwide pandemics—obesity and coronavirus—is undoubtedly putting healthcare systems into an unprecedented level of strain.
ACKNOWLEDGMENTS
We salute the whole nursing team of the ICU of the Arras Hospital for their bravery in this coronavirus disease 2019 crisis. We are proud to be part of this fantastic team!
Footnotes
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Guan WJ, Zhong NS. Clinical characteristics of covid-19 in China. Reply. N Engl J Med 2020; 382:1861–1862 [DOI] [PubMed] [Google Scholar]
- 2.Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: A nationwide analysis. Eur Respir J 2020; 55:2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vincent JL, Lefrant JY, Kotfis K, et al. ; ICON and SOAP investigators; SOAP investigators: Comparison of European ICU patients in 2012 (ICON) versus 2002 (SOAP). Intensive Care Med 2018; 44:337–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 — COVID-NET, 14 States, March 1–30, 2020. Centers for Disease Control and Prevention 2020; 69: 458–464. Available at: https://www.cdc.gov/mmwr/volumes/69/wr/mm6915e3.htm. Accessed May 14, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Intensive Care National Audit and Research Centre (ICNARC): Report on 5578 patients critically ill with COVID-19. Available at: https://www.icnarc.org/Our-Audit/Latest-News/2020/04/17/Report-On-5578-Patients-Critically-Ill-With-Covid-19. Accessed April 20, 2020.
- 6.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ranieri VM, Rubenfeld GD, Thompson BT, et al. ; ARDS Definition Task Force: Acute respiratory distress syndrome: The Berlin definition. JAMA 2012; 307:2526–2533 [DOI] [PubMed] [Google Scholar]
- 8.Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: Guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med 2020; 46:854–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou S, Wang Y, Zhu T, et al. CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR Am J Roentgenol 2020 Mar 5. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Li K, Wu J, Wu F, et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol 2020; 55:327–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000; 894:i– 1–xii, 253 [PubMed] [Google Scholar]
- 12.Fan E, Del Sorbo L, Goligher EC, et al. ; American Thoracic Society, European Society of Intensive Care Medicine, and Society of Critical Care Medicine: An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2017; 195:1253–1263 [DOI] [PubMed] [Google Scholar]
- 13.Spiezia L, Boscolo A, Poletto F, et al. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost 2020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papazian L, Forel JM, Gacouin A, et al. ; ACURASYS Study Investigators: Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010; 363:1107–1116 [DOI] [PubMed] [Google Scholar]
- 15.Guérin C, Reignier J, Richard JC, et al. ; PROSEVA Study Group: Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013; 368:2159–2168 [DOI] [PubMed] [Google Scholar]
- 16.Pirrone M, Fisher D, Chipman D, et al. Recruitment maneuvers and positive end-expiratory pressure titration in morbidly obese ICU patients. Crit Care Med 2016; 44:300–307 [DOI] [PubMed] [Google Scholar]
- 17.Béduneau G, Pham T, Schortgen F, et al. ; WIND (Weaning according to a New Definition) Study Group and the REVA (Réseau Européen de Recherche en Ventilation Artificielle) Network ‡: Epidemiology of weaning outcome according to a new definition. The WIND Study. Am J Respir Crit Care Med 2017; 195:772–783 [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987; 40:373–383 [DOI] [PubMed] [Google Scholar]
- 19.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173:489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993; 270:2957–2963 [DOI] [PubMed] [Google Scholar]
- 21.Ferreira FL, Bota DP, Bross A, et al. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001; 286:1754–1758 [DOI] [PubMed] [Google Scholar]
- 22.De Jong A, Chanques G, Jaber S. Mechanical ventilation in obese ICU patients: From intubation to extubation. Crit Care 2017; 21:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacIntyre NR. Mechanical ventilation in the context of a “bag-in-box” respiratory system. Crit Care Med 2012; 40:1988–1989 [DOI] [PubMed] [Google Scholar]
- 24.Ferretti A, Giampiccolo P, Cavalli A, et al. Expiratory flow limitation and orthopnea in massively obese subjects. Chest 2001; 119:1401–1408 [DOI] [PubMed] [Google Scholar]
- 25.Lemyze M, Mallat J, Duhamel A, et al. Effects of sitting position and applied positive end-expiratory pressure on respiratory mechanics of critically ill obese patients receiving mechanical ventilation*. Crit Care Med 2013; 41:2592–2599 [DOI] [PubMed] [Google Scholar]
- 26.Eichenberger AS, Proitti S, Frascarolo P, et al. Morbid obesity and postoperative pulmonary atelactasis: An underestimated problem. Anesth Analg 2002; 95: 1788–92 [DOI] [PubMed] [Google Scholar]
- 27.Yamane T, Date T, Tokuda M, et al. Hypoxemia in inferior pulmonary veins in supine position is dependent on obesity. Am J Respir Crit Care Med 2008; 178:295–299 [DOI] [PubMed] [Google Scholar]
- 28.Gattinoni L, Coppola S, Cressoni M, et al. Covid-19 does not lead to a “Typical” acute respiratory distress syndrome. Am J Respir Crit Care Med 2020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honce R, Schultz-Cherry S. Impact of obesity on influenza a virus pathogenesis, immune response, and evolution. Front Immunol 2019; 10:1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jense HG, Dubin SA, Silverstein PI, et al. Effect of obesity on safe duration of apnea in anesthetized humans. Anesth Analg 1991; 72:89–93 [DOI] [PubMed] [Google Scholar]
- 31.Futier E, Constantin JM, Pelosi P, et al. Noninvasive ventilation and alveolar recruitment maneuver improve respiratory function during and after intubation of morbidly obese patients: A randomized controlled study. Anesthesiology 2011; 114:1354–1363 [DOI] [PubMed] [Google Scholar]
- 32.Jaber S, Monnin M, Girard M, et al. Apnoeic oxygenation via high-flow nasal cannula oxygen combined with non-invasive ventilation preoxygenation for intubation in hypoxaemic patients in the intensive care unit: The single-centre, blinded, randomised controlled OPTINIV trial. Intensive Care Med 2016; 42:1877–1887 [DOI] [PubMed] [Google Scholar]
- 33.Collins JS, Lemmens HJ, Brodsky JB, et al. Laryngoscopy and morbid obesity: A comparison of the “sniff” and “ramped” positions. Obes Surg 2004; 14:1171–1175 [DOI] [PubMed] [Google Scholar]
- 34.De Jong A, Molinari N, Sebbane M, et al. Feasibility and effectiveness of prone position in morbidly obese patients with ARDS: A case-control clinical study. Chest 2013; 143:1554–1561 [DOI] [PubMed] [Google Scholar]
- 35.Pelosi P, Croci M, Calappi E, et al. Prone positioning improves pulmonary function in obese patients during general anesthesia. Anesth Analg 1996; 83:578–583 [DOI] [PubMed] [Google Scholar]
- 36.El-Solh AA, Aquilina A, Pineda L, et al. Noninvasive ventilation for prevention of post-extubation respiratory failure in obese patients. Eur Respir J 2006; 28:588–595 [DOI] [PubMed] [Google Scholar]
- 37.Winkelman C, Maloney B. Obese ICU patients: Resource utilization and outcomes. Clin Nurs Res 2005; 14:303–323; discussion 324–326 [DOI] [PubMed] [Google Scholar]
- 38.Chiumello D, Cressoni M, Carlesso E, et al. Bedside selection of positive end-expiratory pressure in mild, moderate, and severe acute respiratory distress syndrome. Crit Care Med 2014; 42:252–264 [DOI] [PubMed] [Google Scholar]


