FIGURE 4.
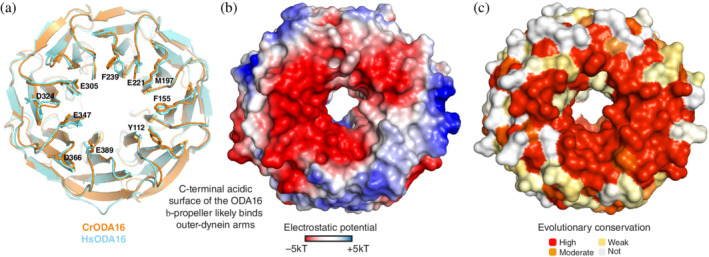
A highly conserved acidic surface patch on the C‐terminal face of ODA16. (a) Superpositioning of Cr‐ and Hs‐ODA16 structures in cartoon representation showing the C‐terminal face of the β‐propeller with 10 completely conserved surface residues displayed as sticks and numbered according to the HsODA16 sequence. The 10 conserved residues likely constitute a common binding site for ODAs. (b) surface representation of ODA16 colored according to electrostatic potential (negatively charged areas are colored red and positively charged areas are colored blue according to the color bar below the figure). The C‐terminal face of ODA16 is mainly negatively charged. (c) Surface representation of the mapping of amino acid conservation onto the ODA16 structure. (a–c) show ODA16 in the same orientation
