Figure 1. Functional heterogeneity of T cells that help B cells.
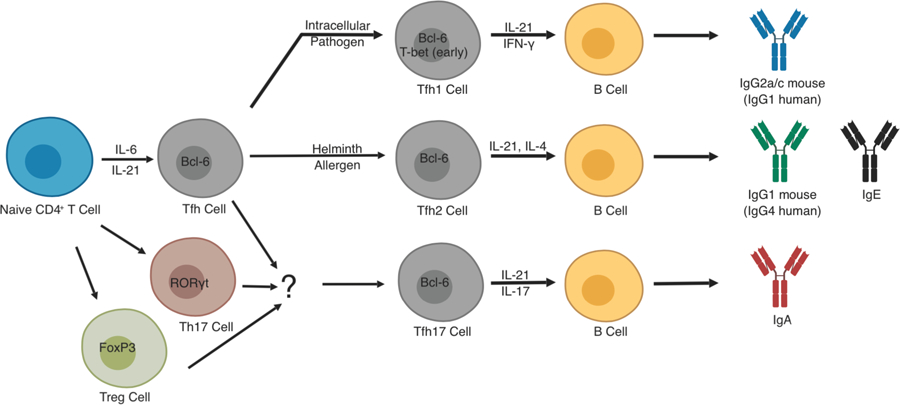
Tfh cells can adopt functional subtypes that correlate with the fate decisions of their T effector counterparts. This is best exemplified for type 1 and 2 immune responses where Tfh cells can adopt Tfh1 or Tfh2 subtypes, respectively. IFN-γ expressed early during a type I immune to virus, for example, will lead to early T-bet expression in Bcl6+ Tfh cells. This leads to IL-21 and IFN-γ co-expression by Tfh1 cells and skews GC B cells to switch to pro-inflammatory IgG2a/c isotypes for aid in clearance of intracellular pathogens. In the case of type 2 infection, Tfh cells adopt a Tfh2 subtype characterized by sequential expression of IL-21 and IL-4 leading to GC B cells isotype switching to IgG1 or IgE for pathogen neutralization and activation of mast cells, respectively. The situation for Tfh17 development is more complex. Current thinking suggests that Tfh17 subsets exist, particularly in Peyer’s patches or other lymphoid tissue associated with mucosal surfaces. These Tfh17 cells are thought to arise primarily from Th17 cells, or perhaps, Treg cells, which can then induce IgA production by GC B cells. All of these subtypes of Tfh cells, Tfh1, Tfh2, and Tfh17 have been implicated in various autoimmune conditions as described in the text.
