Figure 1. Dynamic interactions of Rheb with lysosomal membranes support the activation of mTORC1 signaling.
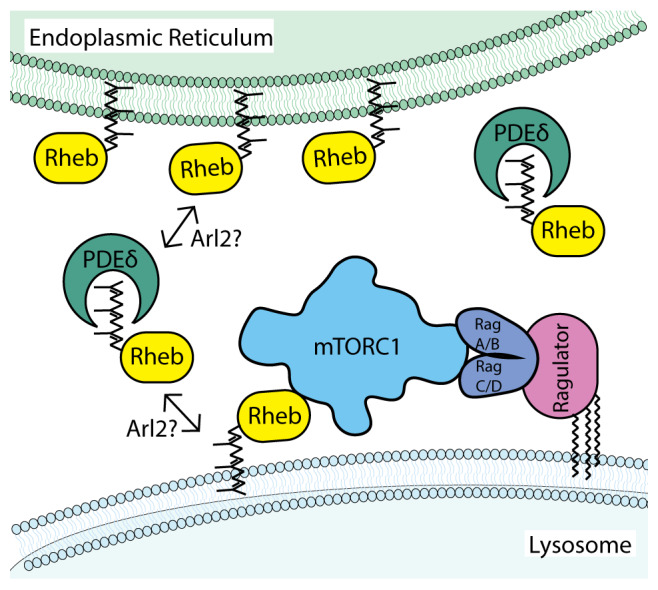
In this model, farnesylation of Rheb is a major determinant of Rheb subcellular localization. The weak membrane association conferred by farnesylation means that steady-state levels of Rheb on lysosomes are low and influenced by additional factors such as sequestration in the cytoplasm by PDEδ and Arl2-dependent release on target membranes. In contrast to Rheb, the Rag GTPases are enriched on lysosomal membranes through interactions with the pentameric Ragulator complex whose LAMTOR1/p18 subunit is both myristoylated and palmitoylated on an N-terminal glycine with two adjacent cysteines 21. Although each of these cysteines represent putative sites of palmitoylation, it remains to be formally established whether they are simultaneously palmitoylated. Furthermore, it was recently reported that LAMTOR1/p18 palmitoylation can be regulated in response to changes in amino acid availability 22. Although simplified in this schematic diagram, mTORC1 is a dimer and can thus potentially engage a total of six GTPases on the surface of lysosomes (two Rag heterodimers that can be made up of different pairwise combinations of RagA or RagB with RagC or RagD plus two Rheb/RhebL1) 1, 23. mTORC1, mammalian target of rapamycin complex 1; PDEδ, δ subunit of phosphodiesterase 6; Rheb, Ras homolog enriched in brain.
