Abstract
Background
Information about incidence, clinical characteristics, and outcomes of HIV-infected individuals with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is scarce. We characterised individuals with COVID-19 among a cohort of HIV-infected adults in Madrid.
Methods
In this observational prospective study, we included all consecutive HIV-infected individuals (aged ≥18 years) who had suspected or confirmed COVID-19 as of April 30, 2020, at the Hospital Universitario Ramón y Cajal (Madrid, Spain). We compared the characteristics of HIV-infected individuals with COVID-19 with a sample of HIV-infected individuals assessed before the COVID-19 pandemic, and described the outcomes of individuals with COVID-19.
Findings
51 HIV-infected individuals were diagnosed with COVID-19 (incidence 1·8%, 95% CI 1·3–2·3). Mean age of patients was 53·3 years (SD 9·5); eight (16%) were women, and 43 (84%) men. 35 (69%) cases of co-infection had laboratory confirmed COVID-19, and 28 (55%) required hospital admission. Age and CD4 cell counts in 51 patients diagnosed with COVID-19 were similar to those in 1288 HIV-infected individuals without; however, 32 (63%) with COVID-19 had at least one comorbidity (mostly hypertension and diabetes) compared with 495 (38%) without COVID-19 (p=0·00059). 37 (73%) patients had received tenofovir before COVID-19 diagnosis compared with 487 (38%) of those without COVID-19 (p=0·0036); 11 (22%) in the COVID-19 group had previous protease inhibitor use (mostly darunavir) compared with 175 (14%; p=0·578). Clinical, analytical, and radiological presentation of COVID-19 in HIV-infected individuals was similar to that described in the general population. Six (12%) individuals were critically ill, two of whom had CD4 counts of less than 200 cells per μL, and two (4%) died. SARS-CoV-2 RT-PCR remained positive after a median of 40 days from symptoms onset in six (32%) individuals, four of whom had severe disease or low nadir CD4 cell counts.
Interpretation
HIV-infected individuals should not be considered to be protected from SARS-CoV-2 infection or to have lower risk of severe disease. Generally, they should receive the same treatment approach applied to the general population.
Funding
None.
Introduction
An outbreak of pneumonia of unknown origin was first reported in Wuhan, China, on Dec 31, 2019. After a week, the cause had been identified as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 With a persistently increasing number of cases of COVID-19 worldwide, WHO declared a pandemic on March 11, 2020.2 Spain has been one of the most affected countries worldwide with 203 715 confirmed cases as of April 30, 2020.3 Particularly, the Community of Madrid has documented the highest number of cases within the country.4
HIV-infected individuals might be at an increased risk of SARS-CoV-2 infection or severe disease, especially individuals with comorbidities, lower CD4 cell counts, or unsuppressed HIV RNA viral load.5, 6 Conversely, immunosuppression or regular use of antiretrovirals such as protease inhibitors, nucleoside reverse transfer inhibitors, or non-nucleoside reverse transfer inhibitors (NNRTI) might modify the risk of infection with SARS-CoV-2 and clinical presentation in this population.7, 8, 9, 10, 11 Here, we describe the SARS-CoV-2 infection rate and clinical characteristics of COVID-19 among adults living with HIV.
Methods
Study design and participants
This was an observational prospective study at the Hospital Universitario Ramón y Cajal (Madrid, Spain), a tertiary university hospital with 1100 beds with 2873 adult patients with HIV on regular follow-up at the monographic HIV clinics. We included consecutive HIV-infected individuals aged 18 years or older with a diagnosis of suspected or confirmed COVID-19 as of April 30, 2020. All research was done according to the Declaration of Helsinki and local legislation. The study protocol was approved by our institutional review board (EC 110/20) and patients provided oral informed consent to minimise physical contact with study staff.
Research in context.
Evidence before this study
We searched PubMed using the terms “COVID-19”, “coronavirus disease 2019”, and “HIV” for studies published from Nov 1, 2019, to April 14, 2020, in any language. We found 13 articles about COVID-19 in HIV-infected individuals. These studies included one related to the rate of infection, one case series, two case reports, one about CT features in an HIV-infected individual with COVID-19, and eight epidemiological perspectives regarding HIV response or health care. Controversies exist regarding the role of some antiretrovirals on preventing or treating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, as well as the influence of immune dysfunction on clinical presentation.
Added value of this study
To our knowledge, this is the first study to comprehensively describe the infection rate of COVID-19 in people living with HIV compared with the general population in the same region and the clinical characteristics and outcomes of COVID-19 in a prospective cohort of HIV-infected individuals. As of April 30, 2020, 51 COVID-19 cases were diagnosed among a cohort of 2873 HIV-infected individuals (incidence 1·8% [95% CI 1·3–2·3]). COVID-19 presented similar clinical, laboratory, and radiographical features in HIV-infected individuals compared with reports of the general population. Among HIV-infected individuals, those with COVID-19 had a significantly higher prevalence of comorbidities. Lower CD4 cell counts affected disease severity and viral kinetics. Age-adjusted mortality was higher in our cohort than that described in the general population in the same region.
Implications of all the available evidence
HIV-infected individuals should not be considered protected from SARS-CoV-2 infection or as having lower risk of severe disease. Indeed, those with low CD4 cell counts might have worse outcomes than individuals with restored immunity. Globally, they should receive the same treatment approach as that applied to the general population.
Data collection
Since the beginning of the epidemic, all new suspected or confirmed COVID-19 cases diagnosed at the hospital were marked with a specific alert signal in electronic health records. We detected co-infection with HIV and SARS-CoV-2 by crossing the HIV clinic database and the dataset of individuals with the alert signal of COVID-19. Additionally, physicians who run HIV clinics in the hospital were notified of all COVID-19 diagnoses in their clinic attendees. Furthermore, incident cases diagnosed in other health centres were notified by the patients themselves or their families to their usual treating physician, as is standard practice when any event occurs to patients with HIV who regularly attend the clinic. Data were extracted from electronic health records daily by staff of the Department of Infectious Diseases by use of a standardised data collection form. Recorded variables were age, gender, comorbidities, HIV-specific variables (year of HIV infection diagnosis, nadir and recent [ie, most recent within previous 6 months] CD4 cell counts, recent CD4/CD8 ratio, recent RNA-HIV plasma viral load, and current antiretroviral therapy [ART]), clinical characteristics of COVID-19, baseline blood tests, radiological results, treatment, and outcomes. We used epidemiological reports from the health authorities of the Community of Madrid to compare infection rates between HIV-infected individuals and the general population.4
Laboratory procedures
Laboratory confirmation of SARS-CoV-2 infection was done by qualitative real-time RT-PCR assay of nasopharyngeal swabs, sputum, or lower respiratory tract aspirates only in individuals who were admitted to hospital, health-care workers, other essential services, or on a case-by-case basis in high-risk or individuals who reside in closed institutions such as nursing homes, detention centres, mental health facilities, and children's homes.12 Public health authorities' regulations in Spain did not recommend confirmatory tests in individuals presenting with mild acute respiratory infection.12 Blood tests and chest x-rays were done at the emergency room according to the clinical needs of each patient, as decided by emergency room physicians. All radiological assessments were done by radiologists.
Definitions
Confirmed COVID-19 was defined by positive RT-PCR for SARS-CoV-2 in respiratory samples. Suspected cases were those in individuals with compatible clinical or radiological findings who were diagnosed with COVID-19, but who did not have RT-PCR testing, or whose results were inconclusive. Fever was defined as an axillary temperature of 37·3°C or higher.13 Lymphocytopenia was defined as a lymphocyte count of less than 1·0 cells × 109 per L, thrombocytopenia was defined as a platelet count of less than 150 × 109 per L, and increased alanine aminotransferase was defined as 40 units per L or higher; defined according to laboratory reference levels at the Hospital Universitario Ramón y Cajal. Estimated glomerular filtration rate was calculated with the Chronic Kidney Disease Epidemiology Collaboration formula.14 Severe disease was defined as fever or suspected respiratory infection plus respiratory rate greater than 30 breaths per min, oxygen saturation of 93% or less on room air, or acute severe respiratory distress (acute lung infiltrate in chest imaging and ratio of partial pressure of arterial oxygen to fractional concentration of oxygen in inspired air [PaO2/FiO2] of ≤300).15 Critically ill individuals were those with rapid disease progression and respiratory failure with need for mechanical ventilation or organ failure that needs monitoring in an intensive care unit (ICU).16
Statistical analysis
No sample size was calculated given that all individuals with a diagnosis of COVID-19 were included. Comparisons were assessed by using the Mann-Whitney U test for continuous variables, whereas categorical variables were assessed by χ2 test or Fisher's exact test where appropriate. Correlations were assessed by Spearman correlation coefficients. To establish the factors associated with a diagnosis of COVID-19 in patients living with HIV, we compared baseline characteristics of HIV-infected individuals with a diagnosis of COVID-19 with those of HIV-infected individuals who had a visit in the past 6 months of 2019, before the beginning of the epidemic, extracting data from an anonymised database with updated information about age, nadir CD4 cell count, ART, and comorbidities. We used multivariate logistic regression models to explore the factors associated with COVID-19 diagnosis in the cohort, adjusted for age, gender, nadir CD4 cell counts, and years of HIV infection. Statistical significance was defined as a two-sided p value of less than 0·05. All statistics were done with IBM SPSS Statistics, version 25.0.
Role of the funding source
This study had no funder. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
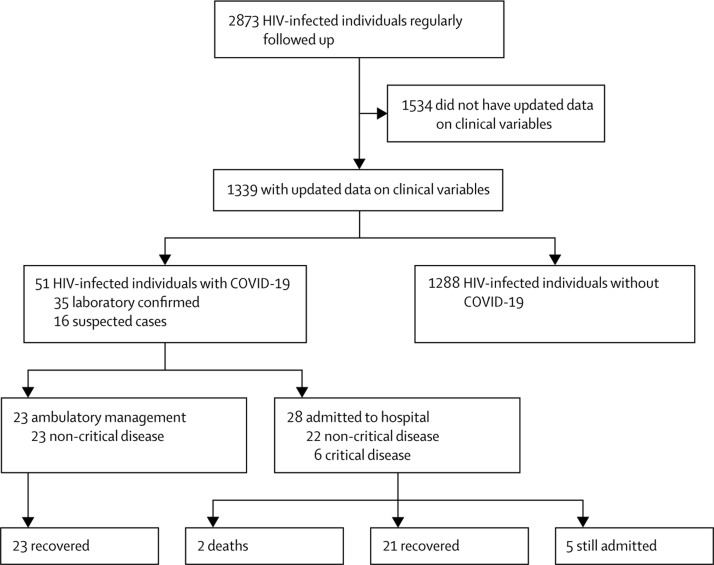
Of 2873 HIV-infected individuals regularly followed up in our clinics, 51 were diagnosed with COVID-19 as of April 30, 2020, resulting in a rate of infection of 1·8% (95% CI 1·3–2·3; figure 1 ). 35 (69%) had a laboratory-confirmed SARS-CoV-2 infection, establishing a confirmed COVID-19 rate of 1·2% (95% CI 0·8–1·7), whereas 16 (31%) were suspected cases. As of the same date, 269 417 cases (61 577 laboratory confirmed and 207 840 suspected) were reported in the Community of Madrid general population, resulting in an overall COVID-19 rate of 4·02% (95% CI 4·01–4·04), and 0·92% (95% CI 0·91–0·93) for confirmed cases. The mean age of HIV-infected individuals with confirmed COVID-19 was slightly lower than the general population (53·6 years [SD 10·0] vs 59·7 years [19·3]) and most (26 [51%]) cases occurred at ages 50–59 years, whereas in the general population, the distribution of COVID-19 was more uniform across age groups (figure 2 ).
Figure 1.
Patient flow diagram
Figure 2.
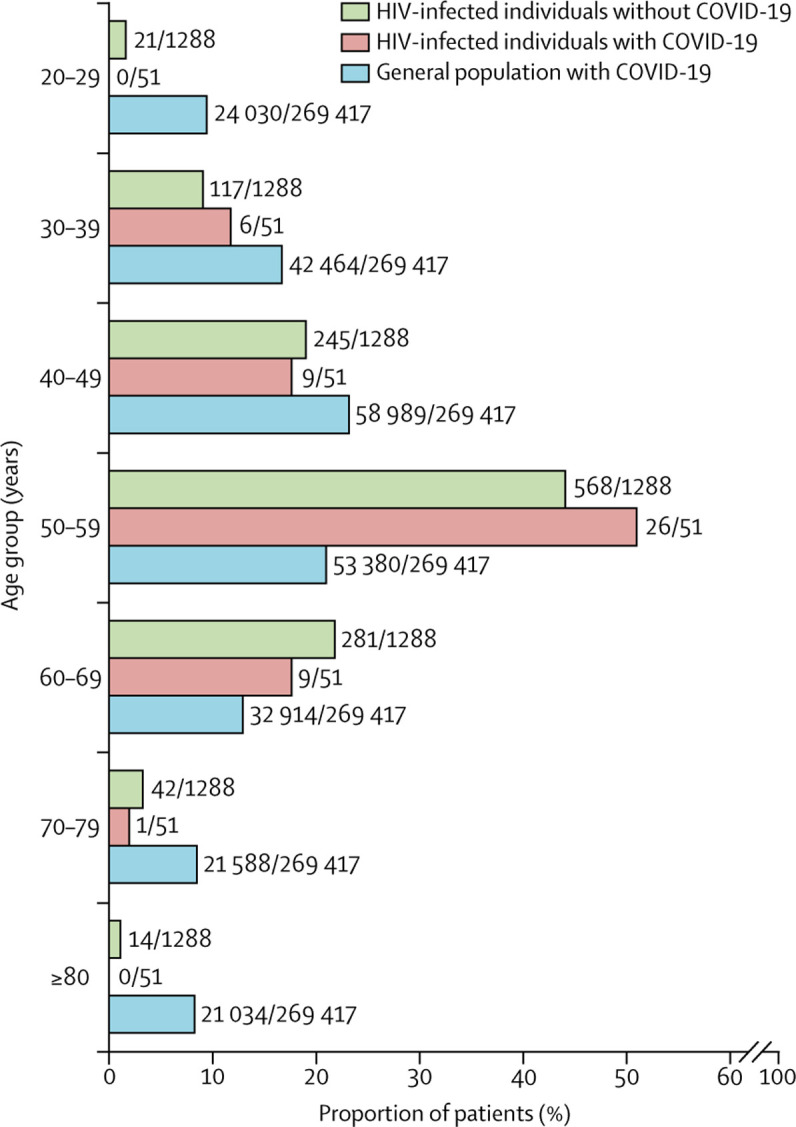
Distribution of HIV-infected individuals with and without COVID-19, and the general population in the Community of Madrid with COVID-19 according to age
We compared baseline characteristics of HIV-infected individuals with (n=51) and without (n=1288) COVID-19. Overall, the age distribution, nadir CD4 cell counts, and the proportion of individuals on ART did not differ between groups (Figure 2, Figure 3 , table 1 ). HIV-infected individuals with COVID-19 had higher median body-mass index (BMI) than those who did not have COVID-19 (25·5 kg/m2 [IQR 22·1–28·0] in patients with COVID-19 vs 23·7 kg/m2 [21·5–26·0] in patients without COVID-19, p=0·021; table 1; appendix p 3), and a higher prevalence of chronic comorbidities (32 [63%] of 51 vs 495 [38%] of 1288, p=0·00059). Regarding ART, a significantly higher proportion of individuals with COVID-19 were receiving tenofovir, either as tenofovir alafenamide (n=36) or tenofovir disoproxil fumarate (n=1), before COVID-19 diagnosis (37 [73%]) than those without COVID-19 (487 [38%], p=0·0036), whereas the use of protease inhibitors or integrase strand transfer inhibitors (INSTIs) was similar in both groups. In the post-hoc analysis of the adjusted multivariable logistic regression model, we found that higher BMI (odds ratio [OR] 1·1 [95% CI 1·0–1·2]), comorbidities (OR 6·2 [95% CI 2·6–14·5]), and use of tenofovir before the COVID-19 pandemic (OR 3·7 [95% CI 1·6–8·7]) were associated with diagnosis of COVID-19. This analysis was repeated with only laboratory-confirmed cases and the results were similar.
Figure 3.
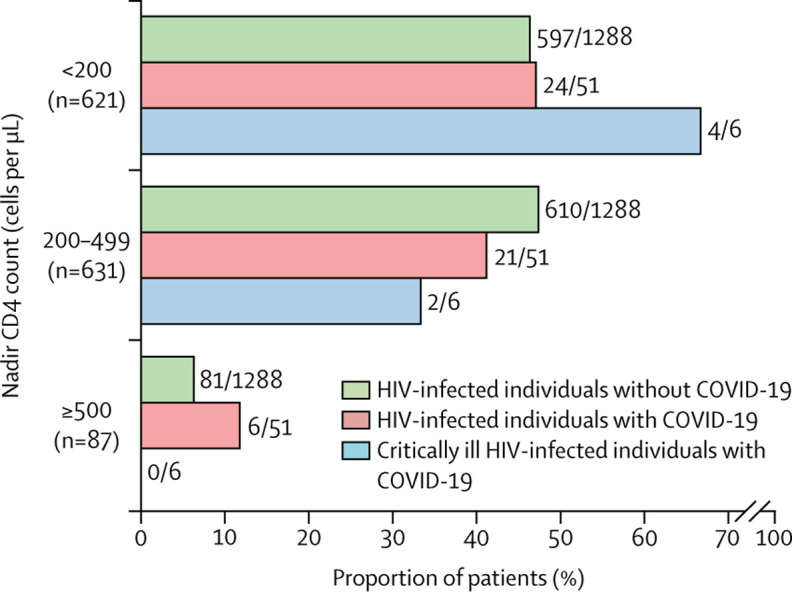
HIV-infected individuals with and without COVID-19 by nadir CD4 cell counts category
Table 1.
Characteristics of HIV-infected individuals with and without COVID-19 diagnosis
| HIV-infected individuals with COVID-19 (n=51) | HIV-infected individuals without COVID-19 (n=1288) | p value | ||
|---|---|---|---|---|
| Age, years | .. | .. | 0·915 | |
| Mean (SD) | 53·3 (9·5) | 53·5 (10·2) | .. | |
| Range | 31–75 | 23–91 | .. | |
| Gender | .. | .. | 0·240 | |
| Female | 8 (16%) | 299 (23%) | .. | |
| Male | 43 (84%) | 989 (77%) | .. | |
| Race | .. | .. | 0·163 | |
| White | 45 (88%) | 1155 (90%) | .. | |
| Black | 0 | 31 (2%) | .. | |
| Asian | 1 (2%) | 4 (<1%) | .. | |
| Latin American | 5 (10%) | 98 (8%) | .. | |
| Body-mass index, kg/m2 | 25·5 (22·1–28·0) | 23·7 (21·5–26·0) | 0·021 | |
| <18·5 | 2 (4%) | 32 (2%) | 0·715 | |
| 18·5–24·9 | 22 (43%) | 518 (40%) | 0·019 | |
| ≥25·0 | 27 (53%) | 311 (24%) | 0·024 | |
| Time since HIV infection diagnosis, years | 19·5 (9·3–28·6) | 22·6 (13·5–28·7) | 0·186 | |
| Nadir CD4 count, cells per μL | 224 (120–437) | 212 (91–330) | 0·182 | |
| <200 | 24 (47%) | 597 (46%) | 1·000 | |
| 200–499 | 21 (41%) | 610 (47%) | 0·396 | |
| ≥500 | 6 (12%) | 81 (6%) | 0·138 | |
| Antiretroviral therapy | ||||
| Any | 51 (100%) | 1284 (>99%) | 1·000 | |
| Protease inhibitors | 11 (22%) | 175 (14%) | 0·578 | |
| NNRTI | 8 (16%) | 269 (21%) | 0·054 | |
| INSTI | 41 (80%) | 707 (55%) | 0·410 | |
| Tenofovir (TAF or TDF) | 37 (73%) | 487 (38%) | 0·0036 | |
| Comorbidities | ||||
| Any | 32 (63%) | 495 (38%) | 0·00059 | |
| Hypertension | 18 (35%) | 102 (8%) | <0·0001 | |
| Diabetes | 7 (14%) | 38 (3%) | 0·0011 | |
| Chronic kidney disease | 6 (12%) | 17 (1%) | 0·00014 | |
| Chronic liver disease | 24 (47%) | 419 (33%) | 0·034 | |
Data are n (%) or median (IQR) unless otherwise specified. INSTI=integrase strand transfer inhibitor. NNRTI=non-nucleoside reverse transcriptase inhibitor. TAF=tenofovir alafenamide. TDF=tenofovir disoproxil fumarate.
Of 51 HIV-infected individuals with COVID-19, the mean age was 53·3 years (SD 9·5), eight (16%) were women, and 32 (63%) had at least one comorbidity. The most common symptoms at hospital consultation were non-productive cough, fever, dyspnoea, and fatigue. The median time from symptom onset to consultation was 6 days (IQR 3–8); 28 (55%) individuals were admitted to hospital and 23 (45%) received ambulatory management (table 2 ).
Table 2.
Characteristics of HIV-infected individuals with COVID-19 by SARS-CoV-2 PCR status at consultation
| Total (n=51) | Confirmed (n=35) | Suspected (n=16) | p value | |||
|---|---|---|---|---|---|---|
| Age, years | .. | .. | .. | 0·346 | ||
| Mean (SD) | 53·3 (9·5) | 53·6 (10·0) | 52·6 (8·8) | .. | ||
| Range | 31–75 | 31–59 | 48–59 | .. | ||
| Gender | ||||||
| Female | 8 (16%) | 5 (14%) | 3 (19%) | 0·694 | ||
| Male | 43 (84%) | 30 (86%) | 13 (81%) | 0·694 | ||
| Current or past smoker | 34 (67%) | 26 (74%) | 8 (50%) | 0·103 | ||
| Comorbidities | ||||||
| Any | 32 (63%) | 23 (66%) | 9 (56%) | 0·547 | ||
| Hypertension | 18 (35%) | 15 (43%) | 3 (19%) | 0·122 | ||
| Cardiovascular disease | 14 (27%) | 10 (29%) | 4 (25%) | 1·000 | ||
| Diabetes | 7 (14%) | 6 (17%) | 1 (6%) | 0·410 | ||
| Chronic kidney disease | 6 (12%) | 3 (9%) | 3 (19%) | 0·363 | ||
| Chronic liver disease | 24 (47%) | 16 (46%) | 8 (50%) | 1·000 | ||
| Chronic respiratory disease | 13 (25%) | 8 (23%) | 5 (31%) | 0·730 | ||
| Cancer diagnosis | ||||||
| On remission | 10 (20%) | 6 (17%) | 4 (25%) | 0·705 | ||
| Active <5 years | 3 (6%) | 3 (9%) | 0 | 0·543 | ||
| Years of HIV infection diagnosis | 19·5 (9·3–28·6) | 16·5 (8·4–25·6) | 25·1 (15·8–31·2) | 0·128 | ||
| Nadir CD4 counts, cells per μL | 224 (101–437) | 174 (128–384) | 168 (90–495) | 0·877 | ||
| Recent CD4 counts, cells per μL | 565 (296–782) | 502 (295–771) | 658 (308–820) | 0·395 | ||
| <200 | 6 (12%) | 4 (11%) | 2 (13%) | 1·000 | ||
| Recent CD4/CD8 ratio | 0·8 (0·8–1·1) | 0·8 (0·5–1·1) | 0·6 (0·4–0·9) | 0·182 | ||
| Last HIV-RNA <50 copies per mL | 50 (98%) | 34 (97%) | 16 (100%) | 1·000 | ||
| Antiretroviral therapy regimen before COVID-19 | ||||||
| Protease inhibitor | 11 (22%) | 7 (20%) | 4 (25%) | 0·723 | ||
| NNRTI | 8 (16%) | 3 (9%) | 5 (31%) | 0·090 | ||
| INSTI | 41 (80%) | 30 (86%) | 11 (69%) | 0·253 | ||
| Tenofovir (TAF or TDF) | 37 (73%) | 24 (69%) | 13 (81%) | 0·503 | ||
| Clinical characteristics | ||||||
| Time from illness onset to hospital consultation, days | 6 (3–8) | 7 (4–8) | 5 (2–11) | 0·676 | ||
| Fever (temperature ≥37·3°C) | 36 (71%) | 25 (71%) | 11 (69%) | 1·000 | ||
| Sore throat | 10 (20%) | 7 (20%) | 3 (19%) | 1·000 | ||
| Cough | 37 (73%) | 24 (69%) | 13 (81%) | 0·503 | ||
| Dyspnoea | 28 (55%) | 21 (60%) | 7 (44%) | 0·367 | ||
| Anosmia or ageusia | 7 (14%) | 4 (11%) | 3 (19%) | 0·664 | ||
| Myalgia | 17 (33%) | 13 (37%) | 4 (25%) | 0·527 | ||
| Headache | 13 (25%) | 8 (23%) | 5 (31%) | 0·730 | ||
| Fatigue | 27 (53%) | 21 (60%) | 6 (38%) | 0·226 | ||
| Diarrhoea | 11 (22%) | 10 (29%) | 1 (6%) | 0·140 | ||
| Nausea or vomiting | 5 (10%) | 3 (9%) | 2 (13%) | 0·643 | ||
| Heart rate, beats per min | 91 (79–107) | 92 (80–110) | 90 (76–106) | 0·490 | ||
| Respiratory rate, breaths per min | 17 (16–20) | 18 (16–22) | 16 (14–16) | 0·00078 | ||
| Mean arterial pressure, mm Hg | 94 (84–104) | 93 (86–103) | 103 (83–116) | 0·414 | ||
| Laboratory results | ||||||
| Patients with available data | 35 (69%) | 29 (83%) | 6 (38%) | 0·0026 | ||
| PaO2/FiO2 | 462 (404–474) | 462 (371–473) | 474 (459–479) | 0·0018 | ||
| <300 | 5 (10%) | 5 (14%) | 0 | 0·566 | ||
| White blood cell count, × 109 per L | 6·8 (4·6–8·4) | 6·4 (4·5–8·5) | 7·0 (4·7–7·9) | 0·450 | ||
| Lymphocyte count, × 109 per L | 1·2 (0·8–1·8) | 1·1 (0·8–1·6) | 1·6 (1·1–4·8) | 0·142 | ||
| Haemoglobin, g/dL | 15·0 (13·0–15·9) | 15·0 (12·7–15·7) | 16·1 (12·9–17·5) | 0·186 | ||
| Platelet count, × 109 per L | 182 (134–229) | 182 (136–226) | 204 (101–275) | 0·704 | ||
| Alanine aminotransferase, U/L | 24 (16–39) | 30 (22–44) | 19 (14–22) | 0·0041 | ||
| Creatinine, mg/dL | 0·9 (0·8–1·1) | 0·9 (0·8–1·1) | 0·9 (0·8–1·28) | 0·260 | ||
| eGFR, mL/min | 90·1 (62·0–97·0) | 90·1 (66·1–97·9) | 85·9 (51·5–98·1) | 0·918 | ||
| Lactate dehydrogenase, U/L | 288 (205–353) | 314 (232–373) | 196 (153–219) | 0·048 | ||
| Troponin I, ng/mL | 0·0 (0·0–0·0) | 0·0 (0·0–0·0) | 0·0 (0·0–0·0) | 0·287 | ||
| D-dimer, ng/mL | 812 (319–1657) | 1056 (324–1903) | 458 (251–458) | 0·047 | ||
| Serum ferritin, ng/mL (n=7) | 972 (366–2791) | 981 (384–3305) | 366 (366–366) | 0·514 | ||
| Procalcitonin, ng/mL | 0·08 (0·04–0·13) | 0·08 (0·04–0·14) | 0·04 (0·01–0·04) | 0·087 | ||
| C-reactive protein, mg/L | 32·1 (7·1–120·6) | 33·3 (8·7–138·0) | 12·5 (1·6–39·7) | 0·0053 | ||
| Interleukin-6, pg/mL (n=10) | 72·7 (30·5–201) | 78·0 (46·7–208·0) | 10·1 (10·1–10·1) | 0·699 | ||
| Interleukin-12, pg/mL (n=10) | 0·7 (0·0–1·32) | 0·9 (0·0–1·5) | 0·5 (0·5–0·5) | 0·741 | ||
| Radiological features | ||||||
| Patients with data available | 38 (75%) | 31 (89%) | 7 (44%) | 1·000 | ||
| Consolidation | 17/38 (45%) | 16/31 (52%) | 1/7 (14%) | 0·0089 | ||
| Interstitial | 11/38 (29%) | 10/31 (32%) | 1/7 (14%) | 0·140 | ||
| Bilateral pulmonary infiltration | 21/38 (55%) | 20/31 (65%) | 1/7 (14%) | 0·031 | ||
| COVID-19 treatment | ||||||
| Any | 39 (76%) | 30 (86%) | 9 (56%) | 0·033 | ||
| Hydroxychloroquine | 30/39 (77%) | 26/30 (87%) | 4/9 (44%) | 0·0017 | ||
| Azithromycin | 19/39 (49%) | 15/30 (50%) | 4/9 (44%) | 0·350 | ||
| Ritonavir-boosted lopinavir | 14/39 (36%) | 12/30 (40%) | 2/9 (22%) | 0·176 | ||
| Tocilizumab | 4/39 (10%) | 4/30 (13%) | 0 | 0·295 | ||
| Systemic corticosteroids | 15/39 (38%) | 13/30 (43%) | 2/9 (22%) | 0·102 | ||
| Outcomes | ||||||
| Respiratory failure | 17 (33%) | 17 (49%) | 0 | 0·00035 | ||
| Intensive care unit admission | 6 (12%) | 6 (17%) | 0 | 0·289 | ||
| Invasive mechanical ventilation | 5 (10%) | 5 (14%) | 0 | 0·167 | ||
| Duration of hospital stay, days | 8 (6–17) | 8 (6–18) | 5 (5–5) | 0·476 | ||
Data are n (%), median (IQR), or n/N (%) unless otherwise stated. eGFR=estimated glomerular filtration rate. INSTI=integrase strand transfer inhibitor. NNRTI=non-nucleoside reverse transcriptase inhibitor. PaO2/FiO2=ratio of partial pressure of arterial oxygen to fractional concentration of inspired oxygen in inspired air. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. TAF=tenofovir alafenamide. TDF=tenofovir disoproxil fumarate.
Laboratory results were available for 35 (69%) HIV-infected individuals with COVID-19. Lymphocytopenia occurred in 15 (43%) of 35 individuals, thrombocytopenia in four (11%), increased alanine aminotransferase in eight (23%), and median PaO2/FiO2 was 462 (IQR 404–474; with five [10%] patients with a ratio <300) at hospital consultation. Notably, 15 (43%) individuals had increased D-dimer concentrations, and the serum cytokine profile showed high interleukin-6 concentrations in seven (70%) of ten analysed cases (table 2). Radiological information was available for 38 (75%) individuals, of whom 17 (45%) had consolidation, 11 (29%) had an interstitial lung pattern, and 21 (55%) had bilateral pulmonary infiltrates.
Besides symptomatic treatment, 12 (24%) of 51 individuals had mild disease and no specific antiviral therapy for COVID-19 was used. By contrast, 39 (76%) received off-label treatment for COVID-19, the most common being hydroxychloroquine (30 [77%] of 39), azithromycin (19 [49%]), and ritonavir-boosted lopinavir (14 [36%], leading to temporal changes in ART), usually used in combination. Additionally, eight (21%) individuals received boosted darunavir, which was part of baseline ART regimens in six individuals. Of note, one (3%) individual received remdesivir in a context of a clinical trial, 15 (38%) received systemic corticosteroids at doses of 1·0–1·5 mg/kg per day of prednisone or equivalent, and four (10%) had off-label use of tocilizumab.
38 (75%) individuals had a mild or moderate disease, whereas it was severe in 13 (25%) patients. Individuals with severe disease had lower lymphocyte counts than those with mild or moderate disease (p=0·049; table 3 ). We assessed the correlation of CD4 cell counts with lymphocyte counts (ρ=0·54; p=0·0036), platelet counts (ρ=0·38; p=0·052), and interleukin-6 (ρ=–0·59; p=0·070). We then compared the characteristics of individuals with recent CD4 counts less than 200 cells per μL (six patients) with those with recent CD4 counts of at least 200 cells per μL (45 patients) and there were no significant differences in clinical characteristics, treatments, or outcomes (appendix p 1).
Table 3.
Characteristics of HIV-infected individuals with COVID-19 according to disease severity
| Mild or moderate COVID-19 (n=38) | Severe COVID-19 (n=13) | p value | ||
|---|---|---|---|---|
| Age, years | 55·4 (46·4–59·8) | 55·5 (51·4–57·5) | 0·762 | |
| Gender | .. | .. | 0·662 | |
| Male | 31 (82%) | 12 (92%) | .. | |
| Female | 7 (18%) | 1 (8%) | .. | |
| Current or past smoker | 22 (58%) | 12 (92%) | 0·038 | |
| Body-mass index, kg/m2 | 25·5 (21·9–28) | 24·3 (22·7–27·8) | 0·931 | |
| Comorbidities | ||||
| Any | 23 (61%) | 9 (69%) | 0·740 | |
| Hypertension | 13 (34%) | 5 (38%) | 0·782 | |
| Diabetes | 6 (16%) | 1 (8%) | 0·662 | |
| Cardiovascular disease | 12 (32%) | 2 (15%) | 0·472 | |
| Time since HIV infection diagnosis, years | 20·1 (8·1–27·9) | 20·5 (10·4–28·7) | 0·795 | |
| Nadir CD4 counts, cells per μL | 232 (100–470) | 190 (109·5–281) | 0·300 | |
| Recent CD4 counts, cells per μL | 668 (313–821) | 422 (287·5–666) | 0·115 | |
| Recent CD4/CD8 ratio | 0·69 (0·48–0·98) | 0·8 (0·5–1·0) | 0·666 | |
| Antiretroviral therapy regimen before COVID-19 | ||||
| Protease inhibitor | 9 (24%) | 2 (15%) | 0·706 | |
| NNRTI | 7 (18%) | 1 (8%) | 0·662 | |
| INSTI | 31 (82%) | 10 (77%) | 0·701 | |
| Tenofovir (TAF or TDF) | 28 (74%) | 9 (69%) | 0·734 | |
| Clinical characteristics | ||||
| Time from illness onset to hospital consultation, days | 6 (2–9) | 7 (2–8) | 0·373 | |
| Fever (temperature ≥37·3°C) | 25 (66%) | 11 (85%) | 0·297 | |
| Sore throat | 8 (21%) | 2 (15%) | 0·657 | |
| Cough | 28 (74%) | 9 (69%) | 0·734 | |
| Dyspnoea | 18 (47%) | 10 (77%) | 0·106 | |
| Anosmia or ageusia | 7 (18%) | 0 | 0·169 | |
| Myalgia | 13 (34%) | 4 (31%) | 0·820 | |
| Headache | 12 (32%) | 1 (8%) | 0·142 | |
| Fatigue | 18 (47%) | 9 (69%) | 0·211 | |
| Diarrhoea | 7 (18%) | 4 (31%) | 0·439 | |
| Nausea or vomiting | 4 (11%) | 1 (8%) | 0·767 | |
| Laboratory results | ||||
| PaO2/FiO2 | 473·7 (462–479·3) | 371·6 (238–448) | <0·0001 | |
| White blood cell count, × 109 per L | 6·5 (4·3–8·4) | 8·1 (4·8–8·5) | 0·555 | |
| Lymphocyte count, × 109 per L | 1·4 (0·9–1·8) | 0·89 (0·7–1·27) | 0·049 | |
| Haemoglobin, g/dL | 15·1 (12·8–15·8) | 15 (12·9–16) | 0·987 | |
| Platelet count, × 109 per L | 178·5 (125·5–227) | 183 (148–234) | 0·674 | |
| Alanine aminotransferase, U/L | 23 (20·5–37·5) | 31 (16–51·5) | 0·319 | |
| Creatinine, mg/dL | 0·93 (0·8–1·0) | 0·94 (0·8–1·2) | 0·775 | |
| eGFR, mL/min | 88·2 (60·7–96·6) | 91·3 (66·1–100·2) | 0·749 | |
| Lactate dehydrogenase, U/L | 233 (196·7–313·5) | 354 (301–476) | 0·003 | |
| D-dimer, ng/mL | 835 (436–2116) | 530 (310–1354) | 0·640 | |
| Serum ferritin, ng/mL (n=7) | 696·8 (380–986) | 2791 (271–4850) | 0·629 | |
| Procalcitonin, ng/mL | 0·05 (0·02–0·11) | 0·09 (0·07–0·18) | 0·109 | |
| C-reactive protein, mg/L | 12·5 (2·4–105·7) | 94·4 (30·9–143) | 0·158 | |
| Interleukin-6, pg/mL (n=10) | 57·7 (10·2–67·4) | 104·7 (35·7–222) | 0·183 | |
| Interleukin-12, pg/mL (n=10) | 0·5 (0·0–1·0) | 0·9 (0·0–1·9) | 0·667 | |
| Radiological features | ||||
| Consolidation | 9 (24%) | 8 (62%) | 0·019 | |
| Interstitial | 6 (16%) | 5 (38%) | 0·121 | |
| Bilateral pulmonary infiltration | 11 (29%) | 10 (77%) | 0·086 | |
| COVID-19 treatment | ||||
| Any | 26 (68%) | 13 (100%) | 0·023 | |
| Hydroxychloroquine | 19 (50%) | 11 (85%) | 0·048 | |
| Azithromycin | 11 (29%) | 8 (62%) | 0·050 | |
| Ritonavir-boosted lopinavir | 6 (16%) | 8 (62%) | 0·0030 | |
| Boosted darunavir | 7 (18%) | 0 | 0·169 | |
| Tocilizumab | 1 (3%) | 3 (23%) | 0·046 | |
| Remdesivir | 0 | 1 (8%) | 0·255 | |
| Systemic corticosteroids | 5 (13%) | 10 (77%) | <0·0001 | |
| Outcomes | ||||
| Respiratory failure | 4 (11%) | 13 (100%) | <0·0001 | |
| Sepsis | 2 (5%) | 9 (69%) | <0·0001 | |
| Critical disease or intensive care unit admission | 0 | 6 (46%) | 0·0028 | |
| Invasive mechanical ventilation | 0 | 5 (38%) | 0·0032 | |
| Death | 0 | 2 (15%) | 0·061 | |
| Recovered | 35 (92%) | 9 (69%) | 0·060 | |
| Duration of hospital stay, days | 8 (6–17) | 8 (6–19) | 0·766 | |
Data are median (IQR) or n (%). eGFR=estimated glomerular filtration rate. INSTI=integrase strand transfer inhibitor. NNRTI=non-nucleoside reverse transcriptase inhibitor. PaO2:FiO2=ratio of partial pressure of arterial oxygen to fractional concentration of inspired oxygen in inspired air. TAF=tenofovir alafenamide. TDF=tenofovir disoproxil fumarate.
During follow-up, six (12%) individuals became critically ill and were admitted to an ICU, and five of them required invasive mechanical ventilation, with a median length of stay at the ICU of 8 days (IQR 6–17). Notably, four (67%) critically ill individuals had nadir CD4 cell counts of less than 200 cells per μL (vs 20 [44%] of 45 non-critically ill patients, p=0·420; figure 3), and two (33%) had recent CD4 cell counts of less than 200 cells per μL (vs four [9%], p=0·141). All critically ill individuals had laboratory-confirmed SARS-CoV-2 infection; and age, gender, comorbidities, ART regimen, and years of HIV infection were not significantly different from patients with non-critical disease. Regarding COVID-19 treatment, critically ill individuals received hydroxychloroquine (four), azithromycin (four), ritonavir-boosted lopinavir (three), systemic corticosteroids (five), tocilizumab (four), or remdesivir (one). Two (33%) of six critically ill individuals died during follow-up after 1 day and 5 days of ICU admission. They had a mean PaO2/FiO2 of 183 (SD 88), C-reactive protein greater than 100 mg/L, and lung consolidations at hospital admission. Furthermore, both were on ART (both on tenofovir, one on protease inhibitors) and had undetectable HIV RNA viral load before admission, with CD4 counts of 137 cells per μL and 636 cells per μL.
44 (86%) individuals had recovered at the time of this analysis and five (10%) were still in hospital. Recovered individuals had higher PaO2/FiO2 (468 vs 319, p=0·002), lower concentrations of C-reactive protein (30·9 ng/mL vs 106·3 ng/mL, p=0·054), and fewer had increased procalcitonin (none of 44 vs two [40%] of five, p=0·013) or lung consolidations (11 [25%] vs four [80%], p=0·0038) at hospital consultation, than individuals who were still in hospital. Notably, previous ART, nadir CD4 cell counts, CD4/CD8 ratio, and the prevalence of comorbidities were not significantly different in recovered versus still-admitted individuals.
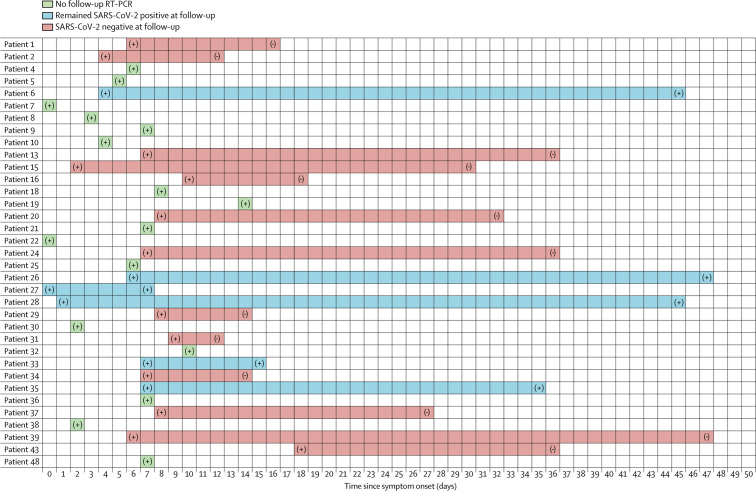
Regarding viral kinetics, 19 (54%) of 35 individuals with laboratory-confirmed SARS-CoV-2 infection had follow-up qualitative RT-PCR assays, which demonstrated viral clearance in 13 (68%) of 19 individuals over a median of 18 days (IQR 7–28) since symptom onset (figure 4 ). Of note, SARS-CoV-2 remained detectable in six (32%) individuals after a median of 40 days (13–45) since symptom onset. Individuals with SARS-CoV-2 positivity at follow-up had longer mean time since diagnosis of HIV infection than those who had negative test results (26·9 years [SD 8·2] vs 16·3 years [9·6]; p=0·023); three (50%) had nadir CD4 counts less than 200 cells per μL, and all had at least one comorbidity. In fact, a greater proportion of the patients with SARS-CoV-2 positivity at follow-up versus those with negative follow-up results had a history of chronic liver disease (three [50%] vs none, p=0·015) and chronic respiratory disease (four [67%] vs four [31%], p=0·025). Four (67%) of the patients with positive follow-up results had severe disease and two (33%) were admitted to intensive care.
Figure 4.
Viral kinetics in HIV-infected individuals with laboratory-confirmed SARS-CoV-2 infection by real-time RT-PCR
Day 0 is the first day of symptoms. + and – indicate SARS-CoV-2 PCR results. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
Discussion
Although the restricted criteria for conducting COVID-19 confirmatory tests in our region preclude us from reaching definitive conclusions,12 we observed a rate of infection in HIV-infected individuals of 1·2–1·8%, similar to that described in a small HIV-infected cohort in Wuhan (0·68–1·0%).7 Compared with the incidence of COVID-19 reported in the general population in our region, the rate of confirmed cases in people living with HIV was similar or slightly higher (1·2% in people with HIV vs 0·96% in the general population), but largely lower after including suspected cases (1·8% vs 4·02%).4 Therefore, we were unable to accurately contrast the rate of COVID-19 in the HIV population with the general population. Our study addresses some of the unknown aspects of COVID-19 in people living with HIV, describing the rate of infection and clinical characteristics of this population in Madrid. To the best of our knowledge, this is the largest cohort of individuals with HIV and SARS-CoV-2 co-infection thus far.
Our findings highlight the increased prevalence of comorbidities in HIV–SARS-CoV-2 co-infected individuals, particularly hypertension, high BMI, diabetes, chronic kidney disease, and chronic liver disease, compared with people with SARS-CoV-2 monoinfection or people with HIV monoinfection.4, 17, 18 This observation might reflect different opportunities for hospital consultation for individuals with comorbidities, since they are usually considered to be at high risk of adverse outcomes. Furthermore, comorbidities are frequently observed in individuals with COVID-19.19
Of clinical importance, days from symptom onset, clinical presentation, laboratory results, and radiographical abnormalities were similar to those described in other cohorts of the general population with COVID-19.13, 20, 21 Our results suggest that HIV-infected individuals with COVID-19 could be treated with the standard of care that is being applied for the general population.22 Most of our patients received hydroxychloroquine, usually in combination with azithromycin or ritonavir-boosted lopinavir, without significant adverse events. Four (11%) individuals received an immunomodulatory drug such as tocilizumab and one was included in a phase 3 clinical trial of remdesivir.
In this study, mortality was 4%, which is lower than that reported in the general population in the Community of Madrid (20%).4, 21 However, in the age strata of 50–59 years, the mortality of our cohort is double that of the mortality in the general population (8% vs 4%).4, 21 Despite the low mortality rate, 25% of HIV-infected individuals with COVID-19 had severe disease and 12% were admitted to an ICU, which is a higher rate than that observed in cohorts of the general population, of whom 17–21% have severe disease and 3–5% are admitted to an ICU.4, 18, 20, 21 Taken together, these data suggest that HIV-infected individuals might have worse outcomes than previously speculated.7
Hypothetically, comorbidities could play an important role in COVID-19 outcomes. In a nationwide analysis from China, the presence of comorbidities was frequent among individuals with severe COVID-19, and it has been associated with poorer clinical outcomes in other studies.19, 20 We did not find an association between comorbidities and disease severity, probably because of the small sample size, the overall high prevalence of comorbidities, or the younger age of HIV-infected individuals relative to the general population.
Previous studies suggest that immunosuppression and low CD4 cell counts might protect HIV-infected individuals from developing the cytokine storm observed in patients with COVID-19.7 We did not find an association between nadir CD4 cell counts and COVID-19 diagnosis in people with HIV after adjusting for baseline characteristics. Nonetheless, lower CD4 cell counts correlated with factors associated with disease severity, such as lower lymphocyte or platelet counts, and higher interleukin-6 levels.23, 24 Additionally, we found that people with HIV who had severe COVID-19 had somewhat lower CD4 counts than those with non-severe disease, although the difference was not significant. Two of six individuals admitted to an ICU had CD4 cell counts of less than 200 cells per μL. Thus, these data clarify the role of immunosuppression in COVID-19 and suggest a higher risk of complications in HIV-infected individuals with low CD4 cell counts at the time of SARS-CoV-2 infection, although the cohort size is small.
Controversies exist regarding the role of some antiretrovirals in preventing or treating COVID-19.25 Guo and colleagues7 reported that no cases of COVID-19 had occurred among 199 HIV-infected individuals taking ritonavir-boosted lopinavir or INSTIs, whereas eight of 947 individuals taking nucleoside reverse transcriptase inhibitors plus NNRTIs were infected. In our cohort, we did not observe differences in previous use of NNRTI, INSTIs, or protease inhibitors in individuals with and without COVID-19 diagnosis. Notably, we observed a higher rate of previous tenofovir alafenamide or tenofovir disoproxil fumarate use in HIV-infected individuals diagnosed with COVID-19 than in those without COVID-19. This result is important because in-vitro studies suggested that tenofovir inhibited SARS-CoV-2 polymerase and ongoing trials are assessing the role of tenofovir as a potential strategy for COVID-19 pre-exposure prophylaxis (NCT04334928).8 Given the observational design and the small sample size, this study should not be taken to confirm or rule out either benefit or harm of tenofovir prophylaxis or treatment against COVID-19. However, our findings do not support a protective role at present and it should not be used outside randomised clinical trials.
Furthermore, previous use of tenofovir alafenamide or tenofovir disoproxil fumarate or protease inhibitors (usually darunavir) was not associated with different clinical presentation in terms of inflammatory markers or severity of COVID-19. During admission, 12 individuals (36% of those who received off-label COVID-19 treatment) had their usual ART modified to include ritonavir-boosted lopinavir, expecting both antiretroviral and anti-SARS-CoV-2 effects.26 This modification was done even in individuals who were receiving other protease inhibitors, such as darunavir, because recent studies showed no activity of this drug against SARS-CoV-2 in vitro.27
The kinetics of SARS-CoV-2 in respiratory samples have clinical and epidemiological implications. The median time to viral clearance in individuals who were admitted to hospital was 18 days (IQR 7–28), similar to that described in other cohorts.13, 28 Notably, six (32%) individuals with confirmed SARS-CoV-2 infection remained positive for the virus after a median of 40 days (IQR 13–45). Higher proportions of individuals with severe disease, ICU admission, low nadir CD4 cell counts, and comorbidities remained positive for the virus than those who did not have these characteristics. Thus, these factors could help identify individuals with delayed viral clearance even after clinical improvement.
Our study had several limitations. First, the small number of individuals prevents us from generalising our results, and we cannot definitively establish the role of immune status or the presence of comorbidities in the clinical presentation and outcomes. Second, as stated, there is bias in the rate of infection because local recommendations restricted confirmatory testing. Although we included all the HIV-infected individuals with COVID-19 to the best of our knowledge, the number might have been underestimated because of the low use of tests or misdiagnosis, especially at the beginning of the epidemic. By contrast, HIV-infected individuals might have a higher opportunity for being tested for SARS-CoV-2 infection even in mild or moderate cases, given that they are usually considered a population at high risk of complications. Nonetheless, we might have missed individuals who were assisted in other health centres who did not report a suspected case of COVID-19 to their treating physician. Third, because of the observational design, individuals were managed from a clinical point of view and inflammatory markers were measured only in severe cases, biasing the effect of these variables on outcomes. Fourth, some variables that could have helped in establishing the role of previous ART or immune status, such as the time of undetectable HIV RNA level or treatment adherence, were not adequately assessed.
HIV-infected individuals seemed to be similarly affected by SARS-CoV-2 compared with the general population in terms of clinical presentation, but we can only speculate about the incidence. Notably, comorbidities were risk factors for COVID-19 diagnosis in this population. By contrast, there was no evidence that any specific antiretroviral drug affected COVID-19 severity. Although low CD4 counts were not associated with the incidence of COVID-19, immunosuppression did seem to affect disease severity, and it might be associated with adverse outcomes and viral persistence. Further studies should clarify the effect of immunosuppression on the risk of SARS-CoV-2 infection. It is crucial that HIV-infected individuals are included in investigational anti-COVID-19 strategies to gain insight into the best approach for this population.
Data sharing
The data for this study are available online in the Mendeley data repository.
Contributors
JLC, MJP-E, and PV conceived and designed the study. All the members of the COVID-19 ID Team were responsible for patient inclusion, clinical follow-up, and helped to write the manuscript. MJP-E, CQ, AM, FD, MJV, and PV collected the data. JLC and PV did the analysis and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors critically revised the manuscript and gave final approval for the final version.
The COVID-19 ID Team
Santiago Moreno (Chief), María Jesús Pérez-Elías, Jesús Fortún, Enrique Navas, Carmen Quereda, Fernando Dronda, Santos Del Campo, Rogelio López-Vélez, Javier Cobo Reinoso, José Luis Casado, Ana Moreno, Francesca Norman, Pilar Martín Dávila, José Manuel Hermida, José Antonio Pérez Molina, Begoña Monge, Vicente Pintado, Sergio Serrano-Villar, Matilde Sánchez-Conde, Sandra Chamorro, Rosa Escudero, Francesca Gioia, Belén Comeche, Clara Crespillo, Sabina Herrera, Raquel Ron, Javier Martínez-Sanz, Mario Pons Guillén, María Jesús Vivancos, and Pilar Vizcarra.
Declaration of interests
MJP-E has received grants and personal fees from Gilead Sciences and ViiV-Healthcare (previously GlaxoSmithKline) and grants from Abbott, outside the submitted work. MJV reports grants and personal fees from Gilead and ViiV-Healthcare outside the submitted work. The other authors declare no competing interests.
Contributor Information
COVID-19 ID Team:
Santiago Moreno, Maria Jesús Pérez-Elías, Jesús Fortún, Enrique Navas, Carmen Quereda, Fernando Dronda, Santos Del Campo, Rogelio López-Vélez, Javier Cobo Reinoso, José Luis Casado, Ana Moreno, Franceca Norman, Pilar Martín-Dávila, José Manuel Hermida, José Antonio Pérez Molina, Begoña Monge, Vicente Pintado, Sergio Serrano-Villar, Matilde Sánchez-Conde, Sandra Chamorro, Rosa Escudero, Francesca Gioia, Belén Comeche, Clara Crespillo, Sabina Herrera, Raquel Ron, Javier Martínez-Sanz, Mario Pons-Guillén, María Jesús Vivancos, and Pilar Vizcarra
Supplementary Material
References
- 1.Phelan AL, Katz R, Gostin LO. The Novel Coronavirus Originating in Wuhan, China: Challenges for Global Health Governance. JAMA. 2020;323:709. doi: 10.1001/jama.2020.1097. [DOI] [PubMed] [Google Scholar]
- 2.WHO WHO Director-General's opening remarks at the media briefing on COVID-19—11 March 2020. Geneva: World Health Organization. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020
- 3.Equipo COVID-19 Red nacional de vigilancia epidemiológica. CNE. CNM (ISCIII). Informe sobre la situación de COVID-19 en España. Informe COVID-19 no 27. April 30, 2020 (in Spanish) https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/INFORMES/Informes%20COVID-19/Informe%20n%C2%BA%2027.%20Situaci%C3%B3n%20de%20COVID-19%20en%20Espa%C3%B1a%20a%2030%20de%20abril%20de%202020.pdf
- 4.Servicio de Epidemiología Informe epidemiológico semanal comunidad de Madrid. Semana 18. May 5, 2020 (in Spanish) http://www.comunidad.madrid/sites/default/files/doc/sanidad/epid/informe_epidemiologico_semanal.pdf (May 10, 2020).
- 5.Zhu F, Cao Y, Xu S, Zhou M. Reply to Comments on ‘Co-infection of SARS-CoV-2 and HIV in a patient in Wuhan city, China’. J Med Virol. 2020 doi: 10.1002/jmv.25838. published online April 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu F, Cao Y, Xu S, Zhou M. Co-infection of SARS-CoV-2 and HIV in a patient in Wuhan city, China. J Med Virol. 2020 doi: 10.1002/jmv.25732. published online March 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo W, Ming F, Dong Y. A survey for COVID-19 among HIV/AIDS patients in two districts of Wuhan, China. SSRN. 2020 https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3550029 published online March 13. (preprint). [Google Scholar]
- 8.Jockusch S, Tao C, Li X. Triphosphates of the two components in DESCOVY and TRUVADA are inhibitors of the SARS-CoV-2 polymerase. bioRxiv. 2020 doi: 10.1101/2020.04.03.022939. published online April 5. [DOI] [Google Scholar]
- 9.Park SY, Lee JS, Son JS. Post-exposure prophylaxis for Middle East respiratory syndrome in healthcare workers. J Hosp Infect. 2019;101:42–46. doi: 10.1016/j.jhin.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joob B, Wiwanitkit V. SARS-CoV-2 and HIV. J Med Virol. 2020 doi: 10.1002/jmv.25782. published online March 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao J, Liao X, Wang H. Early virus clearance and delayed antibody response in a case of COVID-19 with a history of co-infection with HIV-1 and HCV. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa408. published online April 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ministerio de Sanidad Gobierno de España. Procedimiento de actuación frente a casos de infección por el nuevo coronavirus (SARS-CoV-2) April 11, 2020. https://www.saludcastillayleon.es/es/covid-19/informacion-profesionales/informacion-epidemiologica/procedimiento-actuacion-frente-casos-infeccion-nuevo-corona
- 13.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO . World Health Organization; Geneva: March 13, 2020. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected.https://www.internationalmidwives.org/assets/files/news-files/2020/03/who-clinical-management-of-novel-cov-march-2020.pdf [Google Scholar]
- 16.Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30198-5. published online March 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li B, Yang J, Zhao F. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020 doi: 10.1016/S1473-3099(20)30198-5. published online March 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan S, Song X, Lin F. Clinical characteristics of coronavirus disease 2019 in Hainan, China. medRxiv. 2020 doi: 10.1101/2020.03.19.20038539. published online March 23. (preprint). [DOI] [Google Scholar]
- 19.Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging. 2020;12:6049–6057. doi: 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan WJ, Ni ZY, Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borobia AM, Carcas AJ, Arnalich F. A cohort of patients with COVID-19 in a major teaching hospital in Europe. medRxiv. 2020 doi: 10.1101/2020.04.29.20080853. published online May 6. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agencia española de medicamentos y productos sanitarios Tratamientos disponibles para el manejo de la infección respiratoria por SARS-CoV-2. May 19, 2020. https://www.aemps.gob.es/la-aemps/ultima-informacion-de-la-aemps-acerca-del-covid-19/tratamientos-disponibles-para-el-manejo-de-la-infeccion-respiratoria-por-sars-cov-2/?lang=en
- 23.Liu B, Li M, Zhou Z, Guan X, Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. 2020;6 doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blanco JL, Ambrosioni J, Garcia F. COVID-19 in patients with HIV: clinical case series. Lancet HIV. 2020;7:e314–e316. doi: 10.1016/S2352-3018(20)30111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choy K-T, Wong AY-L, Kaewpreedee P. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Meyer S, Bojkova D, Cinatl J. Lack of antiviral activity of darunavir against SARS-CoV-2. medRxiv. 2020 doi: 10.1101/2020.04.03.20052548. published online April 8. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun J, Xiao J, Sun R. Prolonged persistence of SARS-CoV-2 RNA in body fluids. Emerg Infect Dis. 2020 doi: 10.3201/eid2608.201097. published online May 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data for this study are available online in the Mendeley data repository.