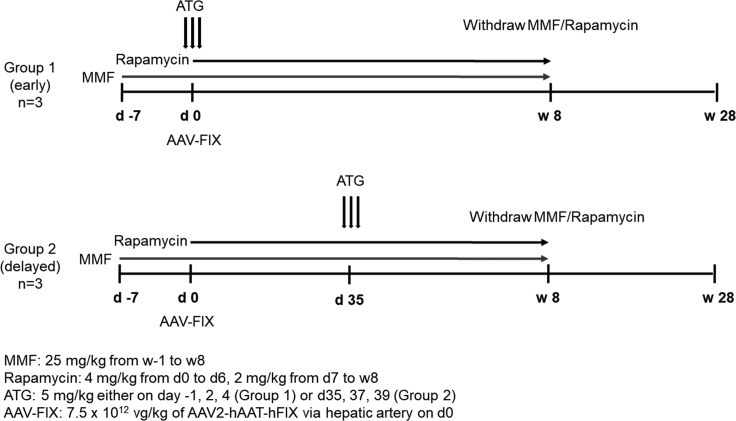
Figure 1.
Experimental Scheme Evaluating the Safety of ATG for Immunosuppression after AAV Gene Therapy
AAV2-hAAT-hFIX at a dose of 7.5 × 1012 vg/kg was administered on day 0. Group 1 animals (early IS) received three doses of ATG around vector administration, whereas group 2 animals (delayed IS) received three doses of ATG around day 35 after vector administration, the approximate time of the onset of T cell cytotoxicity observed in the AAV2 liver-directed clinical trials.6 Both groups received MMF starting 1 week before vector administration and rapamycin starting the day of vector administration. IS was discontinued after 8 weeks from vector administration, and the animals were observed until 28 weeks after vector. Non-linear timescale is employed for clarity.