To the Editor:
As of April 4, 2020, a total of 1,051,635 coronavirus disease 2019 (COVID-19) cases had been reported worldwide, among which 56,985 deaths occurred (5.42%).1 However, to date, no vaccine and no specific antiviral medicine are available to prevent or treat COVID-19. A previous study suggested that cytokine release syndrome could be involved in the pathophysiology of severe or critical COVID-19 cases and frequently results in death.2 Hence, early recognition and effective suppression of the cytokine storm may be life-saving in severe or critical patients. The Fifth Affiliated Hospital of Sun Yat-sen University was the first and, at the time of this report, remains the only designated hospital for management of COVID-19 patients in Zhuhai, China. From January 22 to March 2, we treated 101 patients with COVID-19 in total. Although the previously reported fatality rate for severe and critical patients with COVID-19 was considerable,2 those whom we treated all survived. Despite controversies, we think that timely and appropriate application of glucocorticoid plays a crucial role in the treatment of these patients. Herein, we introduce our clinical experience with corticosteroid administration in these patients for reference and discussion.
During the study period, the Fifth Affiliated Hospital of Sun Yat-sen University was the only designated hospital for the treatment of COVID-19 in Zhuhai, China, and all suspected or confirmed cases in this city were compulsarily admitted to it. All the hospitalized patients were managed by an expert panel consisting of experienced clinicians from pulmonology, critical medicine, infectious diseases, radiology, microbiology, and pathology departments.
Diagnosis of COVID-19 was made on the basis of criteria of the “Diagnosis and Treatment of New Coronavirus-Infected Pneumonia” (Sixth trial version) draft by the National Health Commission of China. Specific IgG antibody had to be tested with the ELISA method before discharge of patients. Furthermore, patients have been followed up and their lung function measured within 1 month of discharge.
This case series was approved by the Institutional Ethics Board of the Fifth Affiliated Hospital of Sun Yat-sen University. Consecutive patients with confirmed COVID-19 admitted from January 22 to March 2, 2020, were enrolled. Oral consent was obtained from patients. We collected and analyzed the clinical data of these patients.
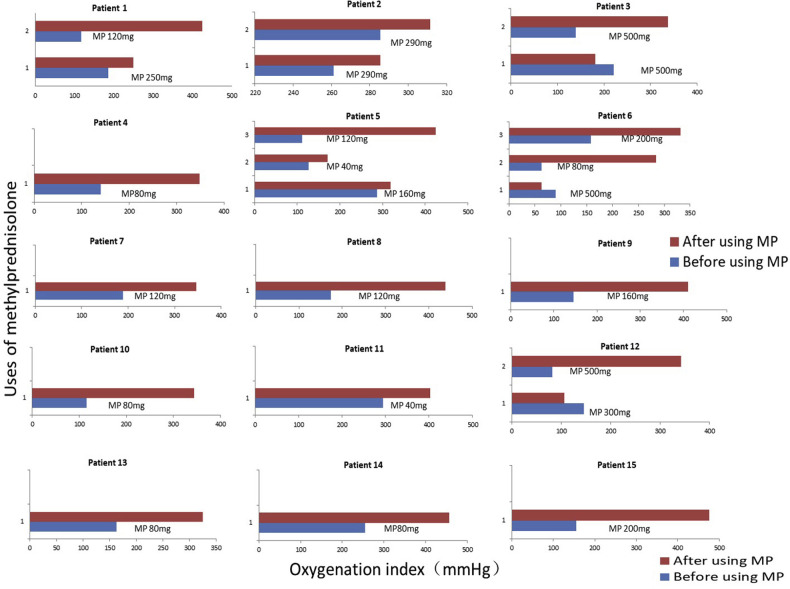
The mean age of the 101 hospitalized patients with confirmed COVID-19 was 45 ± 18.01 years (range, 11 months to 80 years). Forty-seven patients were males, and 69 were from Wuhan (see Table E1 in this article’s Online Repository at www.jacionline.org). Of the 101 cases, 26 were classified as severe or critical (25.74%), and all 26 patients had a recorded ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2 ratio) of less than 300 mm Hg at least 1 time during hospitalization. At least 10 patients had a PaO2/FiO2 ratio of less than 150 mm Hg. All cases have been treated up to discharge standard successfully. We found that the most important treatment experience is timely and appropriate glucocorticoid application in 15 severe and critical patients (with pulse single dosage of 40-500 mg methylprednisolone according to severity, oxygenation index, speed of progression, production of inflammatory factors, body weight, age, and underlying diseases condition, rather than continuous low-dose glucocorticoid for days) (Fig 1 ; see Table E2 in this article’s Online Repository at www.jacionline.org). With this treatment, oxygenation had been improved significantly, and no deaths occurred in these 15 patients. Only 1 of these 15 patients needed mechanical ventilation for 5 days. When observing pulmonary function during the early convalescence phase in patients with COVID-19, we did not find any difference between the 2 groups with or without glucocorticoids (see Table E3 in this article’s Online Repository at www.jacionline.org). We consider that the recovery of lung function in these severe cases received a benefit from using methylprednisolone. Our results also dispelled the worries about the negative impacts of glucocorticoids on virus removal and specific IgG production. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) negative-conversion days were 10.0 ± 5.3 and 10.0 ± 7.9 in patients with and without methylprednisolone therapy, respectively, and there was no statistical difference (see Table E4 in this article’s Online Repository at www.jacionline.org). Besides, the more severe patients with glucocorticoid therapy produced more specific IgG to SARS-CoV-2 than others (see Table E5 in this article’s Online Repository at www.jacionline.org). All patients who received methylprednisolone were examined by pelvic magnetic resonance imaging scans before hospital release as screening for femoral head osteonecrosis, and no abnormal results were observed for any patient. No apparent side effect has been observed during a 1-month follow-up, probably because of the strictly controlled total dosage of methylprednisolone (the highest dosage of methylprednisolone was 1000 mg due to 100 kg bodyweight of the patient).
Fig 1.
The application and effect of methylprednisolone in patients with COVID-19. The abscissa represents the PaO2/FiO2 ratio (mm Hg), and the ordinate represents the times of methylprednisolone injection. The dosage of methylprednisolone was shown in the figure with a unit of milligram. Methylprednisolone was diluted in 50 mL saline and administered intravenously over 30 minutes. The highest single dosage of 500 mg methylprednisolone was administrated according to rapid radiologic progression and high body weight in patient 3 and rapid oxygenation worsening in patient 6 and patient 12. MP, Methylprednisolone. Before using MP: 1 to 2 hours before using methylprednisolone. After using MP: 12 to 24 hours after using methylprednisolone.
The application of glucocorticoid for COVID-19 pneumonia has been controversial, considering the inconclusive clinical evidence and their possible adverse effects. Interim guidance for the management of COVID-19 by the World Health Organization recommends against routinely giving systemic glucocorticoids.3 However, it is well known that glucocorticoids are useful for inhibiting the inflammatory storm via suppression of cytokine levels and proinflammatory gene expression.4 Glucocorticoids thus can diminish the serous exudate at the site of inflammation, reduce tissue edema and injury, and relieve symptoms of inflammation. However, the first report from the autopsy of a patient with COVID-19 revealed that severe serous exudate occurred in the lungs, which was in accordance with the decrease in the peripheral blood serum albumin level frequently found in critical patients. Therefore, based on pathological changes in patients with COVID-19, glucocorticoids could be useful in critical cases. In addition, there is no specific effective antiviral medicine for the new coronavirus and no effective therapy available for suppressing the inflammatory storm except glucocorticoids in urgent clinical settings. In our study, we found that timely and appropriate application of glucocorticoids could avoid the need for invasive mechanical ventilation and improve the outcomes of critical patients with COVID-19, compared with outcomes in reported studies. In a recently published article,5 Shang et al also introduced a series of high-quality studies about the successful use of glucocorticoids for treating SARS, influenza A viral pneumonia, and severe community pneumonia, respectively.6, 7, 8 These supported the use of glucocorticoids in COVID-19 because they have similar pathology.
Continuous administration of glucocorticoids may suppress the immune system and slow down viral clearance. However, our study indicated that single-dose pulse methylprednisolone (40-500 mg methylprednisolone) had no apparent negative impact on SARS-CoV-2 removal and production of specific IgG while effectively stopping the inflammatory cascade.
Timely and appropriate application of methylprednisolone in severe and critical patients with COVID-19 may improve outcomes and lung function without negative impacts on the production of specific IgG to SARS-CoV-2.
Footnotes
This work was supported by grants from Natural Science Foundation of Guangdong Province of China (Grant No. 2020A1515011147 to J.L.) and Scientific Research Foundation of Guangdong Province (Grant No. A2020176 to X.Z.).
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Contributor Information
Hong Shan, Email: shanhong@mail.sysu.edu.cn.
Jin Huang, Email: hjin@mail.sysu.edu.cn.
Supplementary data
References
- 1.World Health Organization Coronavirus disease 2019 (COVID-19): situation report, 75. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports Available at:
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . World Health Organization; 2020. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance, January 28 2020. Available at: https://apps.who.int/iris/bitstream/handle/10665/330893/WHO-nCoV-Clinical-2020.3-chi.pdf. Accessed June 8, 2020. [Google Scholar]
- 4.Darwish I., Mubareka S., Liles W.C. Immunomodulatory therapy for severe influenza. Expert Rev Anti-infect Ther. 2011;9:807–822. doi: 10.1586/eri.11.56. [DOI] [PubMed] [Google Scholar]
- 5.Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet (London, England) 2020;395:683. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen R.C., Tang X.P., Tan S.Y., Liang B.L., Wan Z.Y., Fang J.Q. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. 2006;129:1441–1452. doi: 10.1378/chest.129.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H., Yang S.G., Gu L., Zhang Y., Yan X.X., Liang Z.A. Effect of low-to-moderate-dose corticosteroids on mortality of hospitalized adolescents and adults with influenza A (H1N1) pdm09 viral pneumonia. Influenza Other Respir Viruses. 2017;11:345–354. doi: 10.1111/irv.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siemieniuk R.A., Meade M.O., Alonso-Coello P., Briel M., Evaniew N., Prasad M. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med. 2015;163:519–528. doi: 10.7326/M15-0715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.