Abstract
Background
Corona Virus Disease 19 (COVID-19) had a worldwide negative impact on healthcare systems, which were not used to coping with such pandemic. Adaptation strategies prioritizing COVID-19 patients included triage of patients and reduction or re-allocation of other services. The aim of our survey was to provide a real time international snapshot of modifications of breast cancer management during the COVID-19 pandemic.
Methods
A survey was developed by a multidisciplinary group on behalf of European Breast Cancer Research Association of Surgical Trialists and distributed via breast cancer societies. One reply per breast unit was requested.
Results
In ten days, 377 breast centres from 41 countries completed the questionnaire. RT-PCR testing for SARS-CoV-2 prior to treatment was reported by 44.8% of the institutions. The estimated time interval between diagnosis and treatment initiation increased for about 20% of institutions. Indications for primary systemic therapy were modified in 56% (211/377), with upfront surgery increasing from 39.8% to 50.7% (p < 0.002) and from 33.7% to 42.2% (p < 0.016) in T1cN0 triple-negative and ER-negative/HER2-positive cases, respectively. Sixty-seven percent considered that chemotherapy increases risks for developing COVID-19 complications. Fifty-one percent of the responders reported modifications in chemotherapy protocols. Gene-expression profile used to evaluate the need for adjuvant chemotherapy increased in 18.8%. In luminal-A tumours, a large majority (68%) recommended endocrine treatment to postpone surgery. Postoperative radiation therapy was postponed in 20% of the cases.
Conclusions
Breast cancer management was considerably modified during the COVID-19 pandemic. Our data provide a base to investigate whether these changes impact oncologic outcomes.
Keywords: Breast cancer, Breast surgery, Chemotherapy, COVID-19, Radiation therapy
Highlights
-
•
Management of breast cancer patients was modified during the pandemic.
-
•
Waiting time increased during the pandemic in 20% of the institutions.
-
•
A workload reduction of ≥50% was reported in 1/3 and relocation of the centres in 13%.
-
•
It is unknown whether these changes will affect outcome of breast cancer patients.
1. Introduction
On January 30, 2020, the World Health Organization (WHO) declared the Corona Virus Disease 19 (COVID-19) pandemic caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), a public health emergency of international concern [1]. Less than 4 months later, more than 5 million cases of SARS-CoV-2 infection and more than 350.000 casualties due to COVID-19 were reported worldwide [2]. While SARS-CoV-2 infection passes asymptomatic in an unknown proportion of infected persons, some will develop COVID-19. Of those who require hospitalization, 5–12% will be admitted to an intensive care unit (ICU) [3,4]. The severity of this pandemic put great pressure on healthcare systems worldwide. Countries responded in different ways when reorganising healthcare systems, often with hospitals being specifically assigned for COVID-19 management, and healthcare professionals being appointed to the care of COVID-19 patients. In many instances, this had repercussions on patients affected by other illnesses through a reduction in available facilities, personnel, hospital beds and operating room capacity.
COVID-19 is more severe and lethal among elderly and individuals with underlying comorbidities [5]. In oncology, concerns were raised regarding the risks of SARS-CoV-2 infections related to hospital visits and admissions for treatments, as well as a possible additional risk related to chemotherapy-induced immunosuppression [[6], [7], [8]]. Interestingly, while in general the immune response assists in resolving the infection, in COVID-19 cases an excessive immune response may occur, causing severe lung and systemic pathology [9].
The percentage of COVID-19 among oncologic patients is 2% in China [10]. In Italy, 20% of lethal COVID-19-related complications were reported in patients with active cancer [11]. Consequently, various recommendations and guidelines have been published including the management of breast cancer (BC) [[12], [13], [14], [15], [16]]. Most recommendations are, however, based on expert’s opinion rather than on scientific evidence due to a lack of experience with pandemics. Furthermore, regions have recorded different prevalence of COVID-19, leading to different BC management strategies. In this scenario, the European Breast Cancer Research Association of Surgical Trialists (EUBREAST), a non-profit European association of oncological breast surgeons, initiated an international survey aiming to provide a snapshot of BC management during the second month of the COVID-19 pandemic.
2. Material and methods
A multidisciplinary panel of BC experts, organized by EUBREAST [17], elaborated a 35-question structured questionnaire for a cross-sectional web-based survey among breast centres to investigate changes in BC management during the COVID-19 pandemic. The panel included two gynaecologists (MLG and TK), one breast surgeon (ODG), one medical oncologist (DL), and two radiation oncologists (OKP, PP).
With the support of other international BC societies (appendix A), the survey was distributed to a large network of breast specialists, inviting them to provide only one answer per breast centre. The survey was launched online through a Google™ form on April 18, 2020 and closed on April 28, 2020 [18].
Each healthcare professional completing the questionnaire agreed explicitly to participate in the study. Data from each questionnaire was merged and reported in aggregate into a secure anonymous central database for the analysis. Categorical variables were described as counts and percentages. Differences in categorical variables were analysed using the Fisher’s exact test. Two-sided P values are reported with statistical significance defined as <0.05. Statistical analysis was performed with Microsoft Office Excel 365, version 2020, and GraphPad Prism version 6.0 (GraphPad Software, San Diego, CA, USA).
3. Results
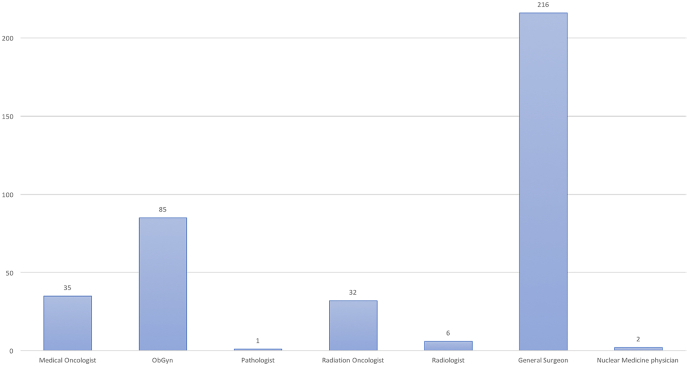
In total, 377 questionnaires from 41 countries were submitted (Appendix A). The majority of responders represented public hospitals and university affiliated hospitals, treating over 300 cases per year. Most replies were registered from breast surgeons, followed by gynaecologists, medical oncologists, radiation oncologists, radiologists, pathologists, and nuclear medicine physicians (Fig. 1). All questions and answers are listed in Appendix B.
Fig. 1.
Specialties of the responders.
3.1. Impact of COVID-19 on breast centres
A total of 129/377 (34.2%) responders reported a reduction in the overall workload of 50% or more and 49/377 (13%) indicated that breast care was relocated.
3.2. Time from diagnosis to initiation of treatment
The estimated time interval between diagnosis and treatment initiation prior to and after the COVID-19 pandemic is shown in Table 3, showing a significant increase.
Table 3.
Time from diagnosis to initiation of treatment.
| Prior covid-19 | During covid-19 | P value | |
|---|---|---|---|
| <2weeks | 132 (35%) | 124 (32.9%) | 0.59 |
| 2–4 weeks | 219 (58.1%) | 149 (39.5%) | 0.0001 |
| >4 weeks | 26 (6.9%) | 104 (27.6%) | 0.0001 |
| Total responses | 377 | 377 | – |
3.3. SARS-CoV-2 screening
PCR testing policies for SARS-CoV2 in women without clinical nor radiological suspicion of SARS-CoV-2 infection varied widely, with only 168/377 (44.8%) institutions performing routine COVID-19 testing before initiation of BC treatment. Among those, 27.1% performed the PCR test before surgery, 5.6% before the first visit to the hospital and 2.4% before chemotherapy, the remaining performing PCR testing before surgery as well as chemotherapy. Only 2% of responders perform the screening by thoracic CT-scan, blood analysis, or preceding radiation therapy. Most responders mentioned that policies concerning testing protocols evolve rapidly.
3.4. Treatment of SARS-CoV-2 positive BC patients
Sixty-five/377 (17.2%) responders treated SARS-CoV-2 positive BC patients, of which 20 treated more than 5 cases.
3.5. Risks of COVID-19 during treatment
Two-hundred and fifty-two/377 (67%) responders considered chemotherapy as being riskier for developing severe COVID-19-related complications compared to surgery and radiation therapy. A total of 95/377 (25.2%) responders considered the risk of severe COVID-19-related complications to be comparable between surgery and chemotherapy. Only 27/377 (7%) of the responders considered the risk of contracting SARS-CoV-2 during surgery higher than during radiation or chemotherapy. The reported cases of patients diagnosed with SARS-CoV-2 during BC treatment or within 14 days following treatment are 10%, 7% and 4% for chemotherapy, surgery and radiation therapy, respectively.
3.6. Primary surgery versus primary systemic therapy
Two-hundred eleven/377 (56%) responders indicated that the indications for primary systemic therapy (PST) were modified, with 140/377 (40%) indicating to treat fewer BC patients with PST during the COVID-19 pandemic compared to before. The number of centres choosing PST as primary treatment for less than 10% of their patients increased from 10.3% to 23.3% (Table 1).
Table 1.
Primary systemic therapy before and during COVID-19.
| Rate of primary systemic therapy | Prior COVID-19 | During COVID-19 | P value |
|---|---|---|---|
| <10% | 39 (10.3%) | 88 (23.3%) | 0.0001 |
| 10–20% | 159 (42.2%) | 143 (37.9%) | 0.2645 |
| 21–30% | 127 (33.7%) | 100 (26.5%) | 0.0383 |
| >30% | 52 (13.8%) | 46 (12.2%) | 0.5883 |
Early stage BC management was modified differently according to the molecular subtype: in patients diagnosed with T1cN0 triple negative or T1c/ER-negative/HER2-positive disease, the indication for PST decreased by approximately 10%, while primary surgery as the preferred approach increased from 39.8% to 50.7% and from 33.7% to 42.2%, respectively (p < 0.016) (Table 2).
Table 2.
Primary systemic therapy versus upfront surgery in specific conditions: T1cN0 triple negative and pT1c-ER negative HER2 positive breast cancer.
| Breast cancer subtype | Primary treatment | Before COVID-19 | During COVID-19 | P value |
|---|---|---|---|---|
| T1cN0 triple negative | Chemotherapy | 227 (60.2%) | 186 (49.3%) | 0.002 |
| Surgery | 150 (39.8%) | 191 (50.7%) | ||
| ER negative HER2 positive pT1c | Chemotherapy in combination with anti HER2 therapy | 250 (66.3%) | 218 (57.8%) | 0.016 |
| Surgery | 126 (33.7%) | 159 (42.2%) |
3.7. Modifications in systemic treatment
One hundred eighty-nine/377 (51%) of the responders reported a modification in chemotherapy protocols, with an increased use of G-CSF (11.4%), decreased number of chemotherapy cycles (7.7%), increased use of oral chemotherapy (7.4%), prolonged interval between cycles (4.2%) and change of type (4%) or sequence of systemic therapy (2.9%). In the remaining cases, the responses were a combination of the above options.
Two-hundred and fifty-five/377 (68%) responders considered initial endocrine treatment in Luminal A disease with the intent to postpone surgery. Twenty-eight percent would do so only in case of risk factors or comorbidities, 16.2% in patients over 65 years old, 12.8% in all cases, and 1.1% in patients between 50 and 65 years old. In the remaining cases, the decision is made case by case.
3.8. Genomic profiling for assessment of the risk of recurrence
Seventy-one/377 (18.8%) institutions indicated that the use of gene expression profiles was increased in order to verify the need for adjuvant chemotherapy.
3.9. Changes in lymphoscintigraphy
No significant changes in the indication for lymphoscintigraphy for axillary surgery were reported.
3.10. Effect on type of breast reconstruction
Before the COVID-19 pandemic, 163/377 (43.2%) responders preferred an immediate reconstruction with a permanent implant, 37/377 (9.8%) favoured an immediate autologous reconstruction, 32/377 (8.5%) a delayed reconstruction with implant, 20/377 (5.3%) a delayed autologous reconstruction, 106/377 (28.1%) an immediate tissue expander and delayed implant and 19/377 (5%) an immediate tissue expander and delayed autologous reconstruction. Most responders expect that the COVID-19 pandemic will affect the choice of reconstruction both for the physicians (178/377 (47.1%)) and for the patients (202/377 (53.5%)).
3.11. Changes in radiation therapy practice
Only 0.5% of responders considered radiation therapy as the major risk factor for COVID-19, compared to surgery or chemotherapy. Over 50% of the breast centres did not modify their radiation therapy schedule. Eighty-three/377 (22.6%) postponed radiation therapy for low risk patients. In 60/377 (15.9%) and 28/377 (7.4%) institutions, fractionation was revised to either a moderate or extreme hypofractionation regimen, respectively. In 8/377 (2.1%) centres, radiation therapy was relocated from a hospital-based to an office-based procedure (Fig. 2).
Fig. 2.
Changes in radiation therapy schedules.
4. Discussion
Our survey gathered responses from a large number of BC centres mainly across Europe in a very short time period and displays an international overview of BC management during the COVID-19 pandemic. The majority of the responders work in large institutions and university affiliated hospitals and treat a large volume of BC patients, fulfilling the requirements set by EUSOMA and endorsed by ECCO [19].
The heterogeneity of our results revealed that presently there is no “one size fits all” approach to delivering BC care during this pandemic. Major disparities in clinical practice underline the complex management of many different clinical and organisational situations and call for consensual evidence-based guidelines. As of now, various recommendations have been published [[12], [13], [14], [15], [16],20,21,23]. However, these recommendations derive mostly from opinions of expert panels and cannot rely on solid data due to the novelty of SARS-CoV-2 and COVID-19.
COVID-19 represents a challenge in oncology for several reasons. Cancer care is of high importance, irrespective of the circumstances. At the occurrence of a major event like the current pandemic, the necessity to triage rises, with the oncological component of prioritisation guided by the tumour type and biological aggressiveness. During the time of the survey, the massive reorganization of healthcare facilities reduced the accessibility of hospitals, specifically operating rooms, for non-infected patients. The risk of progression or death due to progression following delayed cancer care has to be balanced with the risk of contracting SARS-CoV-2 and possible subsequent progression to COVID-19, risking its associated complications and lethality. Consequently, approaches needed to be adapted not merely based on tumour- and patient-related characteristics but also on the prevalence of SARS-CoV-2, the type of organization of healthcare, and the availability of hospital infrastructure and healthcare professionals. However, it remains unknown to date whether delaying cancer treatment, and therefore potentially compromising the prognosis, or delivering a timely treatment in the time of the pandemic with its associated risks, is to be preferred. Overall, this equipoise is the primary concern among our responders.
The American College of Surgeons defines cancer patients as high-priority [20]. Accordingly, the Royal College of Surgeons defines cancer patients and patients with acute diseases as the two groups of patients that should continue to receive surgical care [21]. Twenty-five percent of the responders of our survey are working in areas with a SARS-CoV-2 prevalence of ≥400 cases/100,000 population at the time of the survey. Thirteen percent of the breast centres’ activities were relocated and a much larger impact on the workload of the centres was reported, resulting in a prolongation of the waiting times from 6.9% ≥ 4 weeks before to 27.6% during the pandemic.
In order to maintain an efficient clinical activity, breast centres are reorganising, including prioritisation and triage, the creation of “clean” hospital sites for breast surgery, and virtual multidisciplinary meetings [22,23]. In order to preserve hospital resources, routine breast screenings as well as routine examinations of BC patients in follow up or under adjuvant endocrine therapy have been temporarily suspended, or alternatively, managed through telemedicine [13,24,25]. To reduce infection risks during oncological procedures, cancer centres have been typically organized as SARS-CoV-2 free zones with routine screening methods at entry as well as appropriate adoption of personal protective equipment policies, reducing the risks for contamination during hospitalization for any BC intervention.
The survey indicated that there were different policies to screen for SARS-CoV-2 and to treat infected BC patients. The number of asymptomatic SARS-CoV-2 is thought to be of great importance and each visit to a hospital increases exposure and risks significantly for the visiting patients, the personnel and the hospitalized patients. China’s National Health Commission recorded on April 1, 2020, that 130 (78%) of 166 positive cases were asymptomatic [26]. In Wuhan, 41% of 138 patients were thought to have acquired the infection in the hospital [27]. Among the policies adopted for SARS-CoV-2 screening in patients without respiratory symptoms, a broad range of practices in both the type of test utilized as well as in the criteria used for patient selection was reported, both rapidly evolving over time, for example with increasing availability of test kits [28]. The most effective screening method however, still has to be defined and might differ based on local prevalence of SARS CoV-2. The uncertainty related to the accuracy of screening instruments for SARS-CoV-2 in asymptomatic persons may explain the adoption of a more careful approach even for patients without confirmed infection. When compared to surgery or radiation therapy, the majority of responders considered chemotherapy to be associated with the highest risks for infection with SARS-CoV-2 and progression to COVID-19. This led to a reduced use of PST in favour of a higher rate of upfront surgical treatment. Interestingly, none of the published guidelines recommend upfront surgery over PST as a general rule, instead just as a consideration in certain cases [16]. The possible detrimental effects of chemotherapy on SARS-COV-2 infection, and vice-versa, are also reflected in an increased use of multigene expression-based tests to select patients for whom adjuvant chemotherapy might be avoided. Furthermore, both an increase in GCS-F use and a prolonged interval between chemotherapy regimens were reported. This finding highlights the lack of a consensual way how to adapt chemotherapy regimens during a pandemic. In hormone receptor positive patients, endocrine therapy is not expected to compromise infective receptiveness or the immune response.
Over 50% did not alter their radiation therapy schedule during the COVID-19 pandemic, as radiation therapy is assumed to not have systemic effects on the patient and thereby no increased risks related to COVID-19. However, although radiation therapy may not in and of itself increase a patient’s susceptibility, daily visits to the hospital and interactions with healthcare professionals, and potentially with other patients, increases exposure risks. As such, hypofractionation should be considered as protective by reducing the number of hospital visits.
Surprisingly, in our survey, almost 20% of the responders treated SARS-COV-2 positive BC patients, while international guidelines recommend to suspend anticancer therapy in COVID-19 patients following the supposed association between anticancer treatment and severe complications of COVID-19 [13]. Given Hyppocrate’s oath “first do no harm”, it seems crucial to reinforce the concept of screening patients prior to initiating a surgical or a systemic treatment.
Finally, the responders were asked about their “preferred choice” of breast reconstruction after mastectomy. Whereas the optimal surgical solution depends to a great extent on patients’ characteristics that most likely remain unchanged during the pandemic, the responders reported that the COVID-19 pandemic affects the choice of reconstruction both for the physicians (in 47.1% of the cases) and for the patients (in 53.5% of the cases). It can be assumed that due to COVID-19 associated risks, extrinsic factors such as reduced operating time or recovery after surgery may impact the choice of the reconstructive technique even at the risk of impaired aesthetic outcome. Further studies will show, whether the type of reconstruction really changed during the pandemic.
As an additional finding, we noticed that a number of evidence-based improvements remain to be implemented in current daily practice. Gene-expression profiles are proven to help selecting patients in whom adjuvant chemotherapy can be safely avoided but are used in only a minority of the breast centres. Moderately hypofractionated radiation therapy can be considered standard for more than 10 years, with extreme hypofractionation being introduced very recently, reducing the burden of a protracted treatment series while maintaining outcomes, yet even under the COVID-19 pandemic many centres stick to conventional fractionation. For both examples, about 20% of the replies mentioned an increased use, at least for the duration of the pandemic. Thereby, the pandemic might have a positive collateral effect.
This survey provides a precise snapshot in time, in a field that is rapidly evolving in a non-synchronous way among healthcare organizations. However, all breast centres had access to the same guidelines. Ideally, the survey should be repeated regularly among the same centres, which doesn’t seem feasible.
The strengths of our study are the very short time in which the survey was conducted, its multidisciplinarity, the high number of replies, the large number of countries represented, the involvement of many breast-disease related societies and, last but not least, the fact that this is the first survey aimed to analyse the current practices of breast specialists in the challenging field of BC management during the COVID-19 pandemic.
5. Conclusion
This large international survey among breast cancer centres showed that the COVID-19 pandemic affected management of BC patients, including treatment modifications, longer waiting times and increased use of genomic profile analysis. The fast and high response rate reflects both the significance of the topic, and the eagerness of physicians who are managing BC patients to compare practices during the COVID-19 pandemic. Future investigations will demonstrate whether these changes affected patients’ outcomes.
Declaration of competing interest
The authors declare no conflict of interest related to the publication.
Acknowledgments
The authors would like to thank all the breast societies listed in Appendix A, who have helped us in carrying out the research and strongly supported our project, divulgating this initiative among their members.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2020.05.006.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not for profit sectors.
Ethical approval
Not applicable (the approval was not required).
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.https://www.who.int/westernpacific/emergencies/covid-19 10 May 2020.
- 2.https://www.worldometers.info/coronavirus/?utm_campaign=instagramcoach1? 10 May 2020.
- 3.Phua J., Weng L., Ling L. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8:506–517. doi: 10.1016/S2213-2600(20)30161-2. https://doi: 10.1016/S2213-2600(20)30161-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G., Zangrillo A., Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region. Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. https://doi: 10.1001/jama.2020.5394 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verity R., Okell L.C., Dorigatti I. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30243-7. https://doi: 10.1016/S1473-3099(20)30243-7 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu J., Ouyang W., Chua M.L.K. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in wuhan, China. JAMA Oncol. 2020 doi: 10.1001/jamaoncol.2020.0980. https://doi: 10.1001/jamaoncol.2020.0980 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis M.A. Between Scylla and charybdis - oncologic decision making in the time of covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMp2006588. http://doi: 10.1056/NEJMp2006588 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Cannistra S.A., Haffty B.G., Ballman K. Challenges faced by medical journals during the COVID-19 pandemic. J Clin Oncol. 2020 doi: 10.1200/JCO.20.00858. https://doi: 10.1200/JCO.20.00858 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Tay M.Z., Poh C.M., Rénia L. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020 doi: 10.1038/s41577-020-0311-8. https://doi: 10.1038/s41577-020-0311-8 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai A., Sachdeva S., Parekh T. COVID-19 and cancer: lessons from a pooled meta-analysis. JCO Glob Oncol. 2020;6:557–559. doi: 10.1200/GO.20.00097. https://doi: 10.1200/GO.20.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.4683. https://doi: 10.1001/jama.2020.4683 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic/breast-cancer-in-the-covid-19-era 10 May 2020.
- 13.https://www.asco.org/asco-coronavirus-information/care-individuals-cancer-during-covid-19 10 May 2020.
- 14.https://www.facs.org/covid-19/clinical-guidance/elective-case/breast-cancer 10 May 2020.
- 15.https://www.nccn.org/covid-19/pdf/The_COVID19_Pandemic_Breast_Cancer_Consortium_Recommendations_EXECUTIVE_SUMMARY.pdf 10 May 2020.
- 16.https://www.surgonc.org/wp-content/uploads/2020/03/Breast-Resource-during-COVID-19-3.30.20.pdf 10 May 2020.
- 17.https://www.eubreast.com 10 May 2020.
- 18.https://forms.gle/Kkuh28kBx1KqF2348 10 May 2020.
- 19.Biganzoli L., Cardoso F., Beishon M. The requirements of a specialist breast centre. Breast. 2020;51:65–84. doi: 10.1016/j.breast.2020.02.003. https://doi:10.1016/j.breast.2020.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American College of Surgeons COVID-19: elective triage guidelines for surgical care. 2020. http://www.facs.org/-/media/files/covid19/guidance_for_triage_of_nonemergent_surgical_procedures.ashx Mar. 27. Available:10 May 2020.
- 21.Intercollegiate general surgery guidance on COVID-19 update march 27. 2020. http://www.rcsed.ac.uk/news-public-affairs/news/2020/march/intercollegiate-general-surgery-guidance-on-covid-19-update 10 May 2020.
- 22.Joseph A.O., Joseph J.P., Pereira B. Coronavirus outbreak: reorganising the breast unit during a pandemic. Eur J Surg Oncol. 2020 doi: 10.1016/j.ejso.2020.04.031. https://doi: 10.1016/j.ejso.2020.04.031 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.https://associationofbreastsurgery.org.uk/media/252026/abs-statement-270420.pdf
- 24.Dietz J.R., Moran M.S., Isakoff S.J. Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic. the COVID-19 pandemic breast cancer consortium. Breast Canc Res Treat. 2020 doi: 10.1007/s10549-020-05644-z. https://doi:10.1007/s10549-020-05644-z [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curigliano G., Cardoso M.J., Poortmans P. Recommendations for triage, prioritization and treatment of breast cancer patients during the COVID-19 pandemic. Breast. 2020;52:8–16. doi: 10.1016/j.breast.2020.04.006. https://doi: 10.1016/j.breast.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Day M. Covid-19: four fifths of cases are asymptomatic, China Figures indicate. BMJ. 2020;369:m1375. doi: 10.1136/bmj.m1375. https://doi: 10.1136/bmj.m1375 [DOI] [PubMed] [Google Scholar]
- 27.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.1585. https://doi: 10.1001/jama.2020.1585 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.James W. UK has conducted 18 000 coronavirus tests in 24 hours: PM’s spokesman. 2020. https://www.reuters.com/article/us-health-coronavirus-britain-tests/uk-has-conducted-18000-coronavirus-tests-in- 24-hours-pms-spokesman-idUSKCN21V139 April 13, 10 May 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.