Abstract
Listeria monocytogenes is Gram-positive bacterial pathogen, a causative agent of food poisoning and systemic disease – listeriosis. This species is still susceptible to several conventionally used antibiotics but an increase in its resistance has been reported. For this reason the search for new, alternative therapies is an urgent task. Silver nanoparticles seem to be the promising antibacterial agent. Minimal inhibitory concentration of silver nanoparticles was determined. Sublethal concentrations were used in study of nanosilver effect on cells lysis by estimation of the number of cells surviving the treatment with 0.25 or 0.5 of minimal inhibitory concentrations of silver nanoparticles. Autolysis of isolated peptidoglycan was studied by measuring the absorbance of preparation subjected to nanosilver treatment. Silver nanoparticles effect on L. monocytogenes envelopes permeability was determined by measuring the efflux of cF, DNA and proteins. It was demonstrated that nanosilver enhanced the lysis of L. monocytogenes cells and, to the lesser extent, autolysis of isolated peptidoglycan. The increase in the efflux of carboxyfluoresceine, DNA and proteins was also noted. The obtained results allow to postulate that L. monocytogenes peptidoglycan, constituting the main component of cell wall, is the target of silver nanoparticles activity against this pathogen.
Key words: Listeria monocytogenes, autolysis, peptidoglycan, permeability, silver nanoparticles
Introduction
An ionic form of silver has been used for centuries to cure several diseases which causative agents were bacteria such as Staphylococcus aureus, Klebsiella sp. and Pseudomonas sp. (Rai et al. 2009). It was shown that silver nanoparticles, AgNPs, have higher antibacterial activity than silver ions (Ingle et al. 2008). Nanoparticles are defined as the clusters of atoms of size from 1 to 10 nm with a large surface area to volume ratio, what is proportionally correlated with AgNPs antibacterial activity (Morones et al. 2005). Several studies demonstrated that antibacterial effect of silver nanoparticles is based on their interaction and subsequent damage of cell membranes and on the induction of reactive oxygen species (ROS), including free radicals. The interaction of AgNPs with cell membranes is promoted by their strongly positive zeta potential, which is a difference of an electric potential between the particle and the surrounding solution (Stapsford et al. 2011). This interaction leads to membrane disruption, bacterial flocculation, efflux of cytoplasm, and as a consequence reduction in viability. Formation of ROS is responsible for oxidation, subsequent inactivation and damage of cellular proteins and DNA and peroxidation of lipids (Singh et al. 2008).
It was also proved that AgNPs are active against bacterial biofilms, which are complex bacterial communities resistant to antibiotics and the human immune system. Biofilm resistance is very important and now constitutes a medical challenge as recently the number of infections associated with antibiotic-resistant bacteria living in biofilms has been increased exponentially. These included infections caused by Pseudomonas aeruginosa, the causative agent of nosocomial respiratory tract pneumonia, infections of burn wounds, and chronic lung infections of patients with cystic fibrosis. Biofilm formed by S. aureus also constitutes a very important clinical problem being responsible of e.g. osteomyelitis, periodontitis and chronic wound infections. Well-defined diseases are also caused by biofilms formed by gastrointestinal tract pathogens belonging to Enterobacteriaceae family (for review see Markowska et al. 2013; Wolska et al. 2015). Beside their intrinsic antibacterial activity silver nanoparticles were proved to enhance the effect of conventional antibiotics, such as: vancomycin, amoxicillin, gentamycin, ampicillin, streptomycin in curing bacterial infections (Shahverdi et al. 2007; Birla et al. 2009).
Listeria monocytogenes is a Gram-positive pathogen able to survive in a variety of environments including food, soil and humans. It constitutes very severe problem for food industry because it can survive and multiply even in low temperature; moreover, it forms biofilm and therefore is hard to be eradicated from food production lines. This species is characterized by a unique life mode; it grows in the cytoplasm of the host cell and spreads between cells utilizing actin-based motility (Gray et al. 2006). This pathogen has an ability to cross three human barriers: intestinal, blood-brain and fetoplacental. L. monocytogenes is a causative agent of listeriosis, which can be a fatal infection especially for elder people, immunocompromised individuals, and pregnant women (Alleberger and Wagner 2010). The fatality rate achieves 30%, so this disease represents a major public health concern. Listeriosis in neonates is one of three principal causes of bacterial meningitides. The infections of central nervous system are also described in adults with the mortality rate reaching even 60% (Vázquez-Bolland et al. 2001). L. monocytogenes produces several virulence factors; the major one is listeriolysin O (LLO), a pore-forming toxin belonging to the family of cholesterol-dependent cytolysins that is crucial for escape from vacuole after entry to the mammalian cell (Hamon et al. 2012). The activity of LLO is vital for inducing diarrhea and an inflammatory response after reaching intestinal tract (Barbuddhe and Chakraborty 2009).
The peptidoglycan (murein) constitutes the main compound of L. monocytogenes cell wall and plays a major role in L. monocytogenes pathogenesis (Boneca 2005). Its structure is unusual for Gram-positive bacteria, remaining this of Gram-negative species (e.g. E. coli) because of the presence of partially deacetylated N-acetyloglucosamine residues (Boneca et al. 2007). Another exceptional future of this pathogen is its ability to encode a high number of surface proteins what reflects the ability of L. monocytogenes to survive in a range of diverse environments (Bierne and Cossart 2007). L. monocytogenes is still susceptible to a variety of antibiotic but it should be mentioned that it is intrinsically resistant to a broad spectrum of cephalosporins commonly used in the therapy of many bacterial infections. The resistance to cephalosporins is based on several mechanisms including multidrug transporters and envelope proteins with a detoxification function (Krawczyk-Balska and Markiewicz 2016). In view of expanded resistance to antibiotics, the search for alternative therapies seems to be an urgent task.
The aim of the present study was to investigate the antibacterial effect of silver nanoparticles towards L. monocytogenes in order to identify the cellular target and mechanism of their activity.
Experimental
Materials and Methods
Bacterial strain, growth conditions and reagents used. Reference strain of L. monocytogenes PCM2191 was obtained from Polish Collection of Microorganisms (Institute of Immunology and Experimental Therapy in Wrocław, Poland). The strain was cultivated in tryptone soy yeast extract broth (TSYEB) medium (BTL, Poland) with constant shaking at 37°C. When required, the medium was supplemented with AgNPs and/or solidified with agar (15 g/l). Bacterial stock was stored in freezing solution containing 10% dimethyl sulfoxide (DMSO; v/v). All reagents were ultrapure and were purchased from Sigma, Germany.
Silver nanoparticles. Colloidal water solution of AgNPs was obtained from Nano-Tech (Warsaw, Poland). It contains nanosilver 4N in a concentration of 50 mg/kg, i.e. 50 ppm. The diameter of spherical nanoparticles varied from 2 nm to 35 nm, 70–75% of nanoparticles was within a range of 2–5 nm; their zeta potential was equal to 9.2 mV. Nanoparticles were synthesized by physical method according to the Polish Patent No. 3883399, starting from metallic silver (99.999%) and demineralized water. The detailed characteristics of the preparation used in all experiments was described previously (Chwalibóg et al. 2010).
Determination of MIC for AgNPs and their effect on L. monocytogenes growth and survival. For determination of minimal inhibitory concentration (MIC), the overnight culture of L. monocytogenes was diluted in fresh medium to the density of 2 × 106/ml colony forming units (cfu). The test was performed in 96-well polystyrene plates. To each well the equal volumes of 2-fold concentrated AgNPs suspension and bacterial inoculum were added and the plates were incubated at 37°C for 24 h in static condition. The MIC was determined within the concentration range of AgNPs from 0.5 g/ml to 12 μg/ml at 0.5 intervals. The sample without AgNPs constitutes an experimental control. The MIC value was considered as the lowest concentration entirely inhibiting bacterial growth, according to Clinical and Laboratory Standards Institute (CLSI) instruction. Three independent repetitions were performed. To determine growth and survival curves, the overnight culture of L. monocytogenes was diluted in the fresh medium and incubated until the density of 107 cfu/ml was reached. Then the culture was divided into three equal volumes, AgNPs were added to two of them to a concentration of 0.25 MIC and 0.5 MIC, respectively. The third sample without AgNPs constituted experimental control. The samples were incubated for 24 h, the aliquots were taken for the first 5 h in 1-hour intervals, and finally after 24 h. Their absorbance (A600) was read. Additionally, 0.1 ml aliquots appropriately diluted in saline were plated on solid media and after 24 h of incubation the colonies were counted.
Measurement of autolysis/lysis of L. monocytogenes cells. Overnight culture of L. monocytogenes was diluted in 50 ml of fresh medium to A600 equal to 0.1 and incubated until A600 equal to 0.6 was reached. Bacteria were centrifuged (10 min, 8000 × g) and the pellet, after washing twice in phosphate buffered saline (PBS), was resuspended in Tris-HCl buffer pH 8.0 containing Triton X-100 (0.1%) or lysozyme (20 μg/ml). Both suspensions were divided in three parts, one served as a control without AgNPs, to the reminding two the nanosilver suspensions in a concentration of 0.25 MIC and 0.5 MIC was added. After 60 min of incubation 0.1 ml aliquots appropriately diluted in saline were plated on solid media and after 24 h the colonies were counted.
Measurement of L. monocytogenes peptidoglycan autolysis rate. Overnight culture was divided in three parts, to two of them the suspension of AgNPs in a concentration of 0.25 MIC or 0.5 MIC were added, the third without AgNPs was left as a control. The procedure of peptidoglycan isolation and its absorbance measurement precisely followed those previously described (Kurek et al. 2010).
Estimation of AgNPs effect on permeability of L. monocytogenes envelopes. Overnight culture of L. monocytogenes was diluted to A600 equal to 0.1 and incubated further to A600 equal to 1.0. Then bacteria were spun down, the pellet was rinsed twice with 20 mM phoshate buffer, pH 7.1, and dissolved in this buffer to A600 equal to 0.8. A fluorescent dye, carboxyfluoresceine (cF), was added to the final concentration of 0.54 μM and after incubation at 40°C for 3 min three samples were prepared: the negative control without AgNPs and probes containing AgNPs at a concentration of 0.25 MIC or 0.5 MIC. The additional referential, positive control with 10% DSMO was also included. The samples were incubated at 37°C for 10 min, then the cells were spun down and the fluorescence of supernatants was measured in black 96-wells titration plates using fluorescence reader at wavelengths 490 nm (excitation) and 515 nm (emission), according to Johansen et al. 1997. To measure DNA and proteins release, the cell suspension prepared as described above was split into four parts: the negative control without AgNPs, samples containing AgNPs at a concentration of 0.25 MIC or 0.5 MIC, and positive control containing 100 μg/ml lysozyme. The samples were incubated at 37°C for 1 h, then cells were removed by centrifugation and the absorbance of supernatants at 260 nm (the released DNA) and 280 nm (the released proteins) was measured. This protocol was the modification of the procedure described previously (Markowska et al. 2014).
Statistical analysis The experiments were performed at least three times and every measurement was done in triplicate. The means ± standard deviations were calculated. Statistical significance of the difference between experimental samples was estimated using Student’s test with Graphpad prism (ver. 6.0). p value < 0.05 was considered as statistically significant.
Results and Discussion
MIC of AgNPs was 8 μg/ml, and susceptibility of Gram-positive L. monocytogenes to AgNPs was higher than that observed for one of Gram-negative pathogens Pseudomonas aeruginosa ATCC 10145, for which MIC value was equal to 1 μg/ml (Markowska et al. 2014). The results presented in Fig. 1 showed that neither growth nor survival of L. monocytogenes was substantially diminished in the presence of AgNPs in a concentration of 0.25 MIC or 0.5 MIC. In the subsequent experiments, AgNPs were used in these concentrations.
Fig. 1.
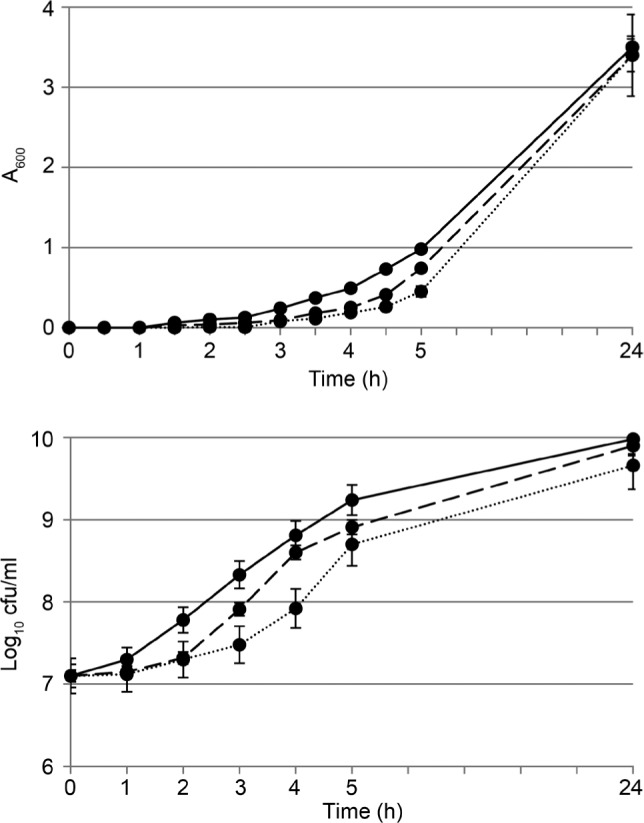
Effect of AgNPs on L. monocytogenes growth (A) and survival (B).
Solid lines – control; dashed lines – 0.25 MIC AgNPs; dotted lines – 0.5 MIC AgNPs. Mean ± SD values of triplicate cultures were shown.
The effect of AgNPs on the autolysis/lysis of L. monocytogenes cells was studied in the cultures treated with nonionic surfactant Triton X-100 or lysozyme. The nonionic detergent Triton-X100 and lysosyme are the commonly used agents for induction of autolysis or lysis of L. monocytogenes cells, respectively (Smith et al. 1991; Popowska et al. 2009). When used in the moderate concentration, they can be applied to study the effect of various substances on their activity. The results of the experiments are presented in Fig. 2. In the control cultures, treated only with lysosyme or Triton X-100, 49% and 89% of cells survived, respectively. The observed killing effect of lysosyme was much weaker than that of Triton X-100 as it had already been presented by Kurek and coauthors (2010). The observed result can be due to the low level of glucosamine acetylation in L. monocytogenes peptidoglycan (Amano et al. 1977). The addition of AgNPs at a concentration of 0.25 MIC resulted in very high, over 1000-fold increase of the lysis of cultures treated with lysozyme or Triton X-100. AgNPs added in a concentration of 0.5 MIC caused further drop in the number of living cells.
Fig. 2.
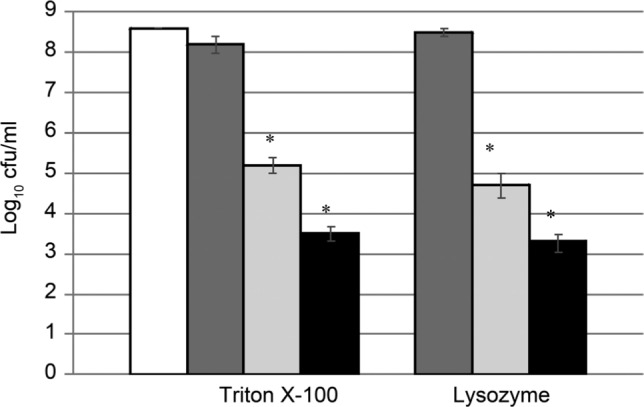
Influence of AgNPs on lysozyme- or Triton X-100-induced autolysis/lysis of L. monocytogenes.
White bar – control, buffer only; dark grey bar – lysozyme or Triton X-100; light grey bar – lysozyme or Triton X-100 + 0.25 MIC AgNPs; black bar – lysozyme or Triton X-100 + 0.5 MIC AgNPs. The results are mean of three independent experiments with every measurement done in triplicate ± SD. Statistically relevant differences (p < 0.05) were marked with asterisks.
The addition of nanosilver to peptidoglycan also caused the enhancement of peptidoglycan autolysis; however, this effect was not as pronounced as the effect observed for the whole cells. The drop in absorbance (A600) of the control peptidoglycan sample was 33% after 2 h incubation in the buffer. In samples treated with AgNPs at the concentration of 0.25 MIC or 0.5 MIC the observed drop was 49% and 53%, respectively. Only the last value was statistically relevant, as it was shown in Fig. 3. It can be speculated that the AgNPs enhanced the ability of autolysins – peptidoglycan hydrolyzing enzymes which catalyse polymer destruction (Rice and Bayles 2008). Five L. monocytogenes autolysins have been identified (Popowska 2004); however, the analysis of the bacteria genome revealed the presence of more than twenty proteins with the putative peptidoglycan hydrolase domains (Bierne and Cossart 2007).
Fig. 3.
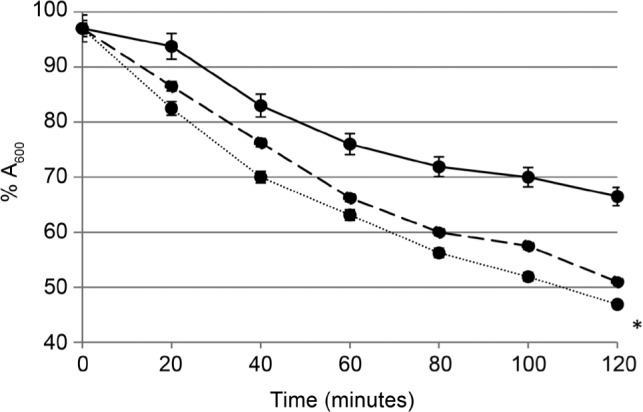
Autolysis of isolated L. monocytogenes peptidoglycan in the presence of AgNPs.
Absorbance A600 at time 0 was considered as 100%. Solid line – control; dashed line – 0.25 MIC AgNPs; dotted line – 0.5 MIC AgNPs. The results are mean of three independent experiments with every measurement done in triplicate ± SD. Statistically relevant difference (p < 0.05) was marked with an asterisk.
The effect of AgNPs on L. monocytogenes cells permeability was estimated by two methods. First, the efflux of cF dye was measured and after 10 min of treatment with AgNPs at a concentration of 0.5 MIC the efflux of cF was enhanced by 14% in comparison to the control sample, which was 79% relative to the total leakage determined after cell lysis with DMSO (Table I). In the second set of experiments the efflux of macromolecules, DNA and proteins, was studied by measuring respectively A260 and A280 of the supernatants of cultures treated with AgNPs at a concentration of 0.25 MIC or 0.5 MIC, in the control culture, and in the culture treated with lysozyme (positive control).
Table I.
Increase in cF efflux from L. monocytogenes caused by AgNPs.
| AgNPs concentration | % efflux of cF at time (min) | |
|---|---|---|
| 0 | 10 | |
| 0 (negative control) | 72 ± 2 | 79 ± 1 |
| 0.25 MIC | 73 ± 2 | 84 ± 2 |
| 0.5 MIC | 74 ± 2 | 93* ± 2 |
Efflux values are given as percentages relative to the total leakage determined after cell lysis with DSMO (means of three independent experiments with every measurement done in triplicate ± standard deviations are shown). Statistically relevant difference (p < 0.05) was marked with an asterisk.
It was demonstrated that AgNPs in a concentration of 0.5 MIC enhanced DNA efflux by 48% after 60 min of treatment with AgNPs in comparison to control culture (Fig. 4). The efflux of proteins was enhanced by 30% after 60 min exposure to AgNPs at a concentration of 0.5 MIC. The maximal efflux of DNA and proteins caused by 60 min exposure to lysozyme amounted to 171% and 191% of the control sample without AgNPs. The observed enhancement of cF, DNA and proteins efflux as a result of AgNPs treatment points to the damage of cell wall. It was previously demonstrated that AgNPs are able to cover cells surface and to induce the formation of the hollows in cell envelopes what can results in the enhancement of cell permeability (Chwalibóg et al. 2010). In turn, AgNPs, when adsorbed on the cell surface, modify membranes potential what stimulates nanoparticles transport to the cytoplasm (Morones et al. 2005; Marambio-Jones and Hoek 2010).
Fig. 4.
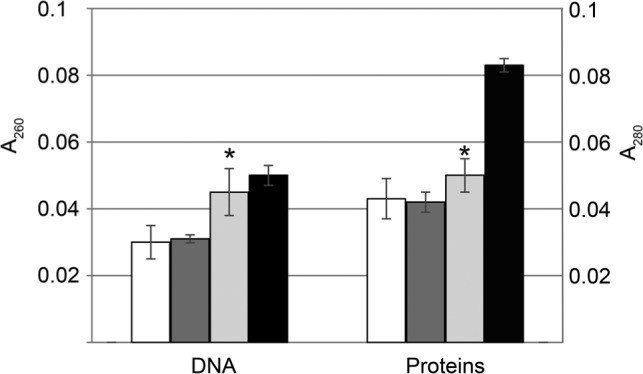
Effect of AgNPs influence on the efflux of DNA and proteins from L. monocytogenes.
White bars – control, no AgNPs added; dark grey bars – 0.25 MIC AgNPs; light grey bars – 0.5 MIC AgNPs; black bars – 100 μg/ml lysozyme (positive control). The results are mean of three independent experiments with every measurement done in triplicate ± SD. Statistically relevant difference (p < 0.05) were marked with asterisks.
Until now there have been only few papers describing the effect of silver nanoparticles on L. monocytogenes membranes. Microscopic analysis demonstrated deformation, disintegration and decrease in cell surface roughness of L. monocytogenes treated with silver nanoparticles synthesized by Jatropa curcas (Chauhan et al. 2016). It has also been demonstrated that AgNPs released from nanocomposites can penetrate the cell wall and plasma membrane of L. monocytogenes what results in separation of the cytoplasmic membrane from the cell wall (Tamayo et al. 2014). The results of our group demonstrated that AgNPs caused the decrease in L. monocytogenes cell length even by 50% what may also indicate their interaction with the cell wall (data not shown) (Milczarek 2015). It has been postulated recently that the activity of AgNPs against L. monocytogenes and the other foodborne pathogens make them useful in food industry, particularly in food packaging and food preservation (Patra and Baek 2017).
In conclusion, the original results presented here show that L. monocytogenes peptidoglycan is the target of AgNPs activity. This effect is demonstrated by the increase of cell autolysis and autolysis of the extracted peptidoglycan and also by the enhancement in cell permeability. Interference with L. monocytogenes cell wall integrity and functionality constitutes the important mechanism of nanosilver antibacterial activity towards this Gram-positive pathogen.
Acknowledgments
This research was supported by the Polish Ministry of Science and Higher Education intramural grant 140000/501/86-107429 through the Faculty of Biology, University of Warsaw. The authors are grateful to Nano-Tech for providing AgNPs. We also express many thanks to dr. Magdalena Popowska (Department of Applied Microbiology, Institute of Microbiology, University of Warsaw) for critical reading of the manuscript.
Literature
- Alleberger F, Wagner M.. 2010. Listeriosis: a resurgent foodborne infection. Clin Microbiol Infect. 16:16–23. [DOI] [PubMed] [Google Scholar]
- Amano K, Hayashi H, Araki Y, Ito E.. 1977. The action of lysozyme on peptidoglycan with N-unsubstituted glucosamine residues. Isolation of glycan fragments and their susceptibility to lysozyme. Eur J Biochem. 76:299–307. [DOI] [PubMed] [Google Scholar]
- Barbuddhe SB, Chakraborty T.. 2009. Listeria as an enteroinvasive gastrointestinal pathogen. Curr Top Microbiol Immunol. 337: 173–195. [DOI] [PubMed] [Google Scholar]
- Bierne H, Cossart P.. 2007. Listeria monocytogenes surface proteins: from genome predictions to function. Microbiol Mol Biol Rev. 71:377–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birla SS, Tiwari VV, Gade AK, Ingle AP, Yadav AP, Rai MK.. 2009. Fabrication of silver nanoparticles by Phoma glomerata and its combined effect against Escheriochia coli, Pseudomonas aeruginosa and Staphylococcus aures. Lett Appl Microbiol. 48:173–179. [DOI] [PubMed] [Google Scholar]
- Boneca IG. 2005. The role of peptidoglycan in pathogenesis. Curr Opin Microbiol. 8:46–53. [DOI] [PubMed] [Google Scholar]
- Boneca IG, Dussurget O, Cabanes D, Nahori MA, Sousa S, Lecuit M, Psylinakis E, Bouriotis V, Hugot JP, Giovannini M, et al. 2007. A critical role for peptodoglycan N-deacetylation in Listeria evasion from the host innate immune system. Proc Natl Acad Sci USA. 104:997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan N, Tyaghi AK, Kumar P, Malik A.. 2016. Antibacterial potential of Jatropha curcas synthesized silver nanoparticles against food borne pathogens. Front Microbiol. 7:1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chwalibóg A, Sawosz E, Hotowy A, Szeliga J, Mitura S, Mitura K, Grodzik M, Orlowski P, Sokolowska A.. 2010. Visualization of interaction between inorganic nanoparticles and bacteria or fungi. Int J Nanomed. 5:1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MJ, Freitag NE, Boor KJ.. 2006. How the bacterial pathogen Listeria monocytogenes mediates the switch from environmental Dr. Jekyll to pathogenic Mr. Hyde. Infect Immun. 74:2505–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon MA, Ribet D, Stavru F, Cossart P.. 2012. Listeriolysin O: the Swiss army knife of Listeria. Trends Microbiol. 20:360–368. [DOI] [PubMed] [Google Scholar]
- Ingle A, Gade A, Pierrat S, Sonnichsen C, Rai M.. 2008. Mycosynthesis of silver nanoparticles using the fungus Fusarium acuminatum and its activity against some human pathogenic bacteria. Curr Nanotechnol. 4:141–144. [Google Scholar]
- Johansen C, Verheul A, Gram L, Gill T, Abee T.. 1997. Protamine-induced permeabilization of cell envelopes of Gram-positive and Gram-negative bacteria. Appl Environ Microbiol. 63:1155–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk-Balska A, Markiewicz Z.. 2016. The intrinsic cephalosporin resistome of Listeria monocytogenes in the context of stress response, gene regulation, pathogenesis and therapeutics. J Appl Microbiol. 120:251–265. [DOI] [PubMed] [Google Scholar]
- Kurek A, Grudniak AM, Szwed M, Klicka A, Samluk Ł, Wolska KI, Janiszowska W, Popowska M.. 2010. Oleanolic acid and ursolic acid affect peptidoglycan metabolism in Listeria monocytogenes. Antonie van Leeuwenhoek Int J Gen Mol Microbiol. 97:61–68. [DOI] [PubMed] [Google Scholar]
- Marambio-Jones C, Hoek EMV.. 2010. A review of the antibacterial effects of silver nanomaterials and potential implications. J Nanoparticle Res. 12:1531–1551. [Google Scholar]
- Markowska K, Grudniak AM, Wolska KI.. 2013. Silver nanoparticles as an alternative strategy against bacterial biofilms. Acta Biochim Pol. 60:523–530. [PubMed] [Google Scholar]
- Markowska K, Grudniak AM, Krawczyk K, Wróbel I, Wolska KI.. 2014. Modulation of antibiotic resistance and induction of stress response in Pseudomonas aeruginosa by silver nanoparticles. J Med Microbiol. 63:849–854. [DOI] [PubMed] [Google Scholar]
- Milczarek BE. 2015. Influence of silver nanoparticles on Listeria monocytogenes cell membranes. Ph. D. Thesis Warsaw (Poland): University of Warsaw. [Google Scholar]
- Morones JR, Elechigerra JL, Camacho A, Ramirez JT.. 2005. The bactericidal effect of silver nanoparticles. Nanotechnology. 16: 2346–2353. [DOI] [PubMed] [Google Scholar]
- Patra JK, Baek KH.. 2017. Antibacterial activity and synergistic antibacterial potential of biosynthesized silver nanoparticles against foodborne pathogenic bacteria along with its anticandidal and antioxidant effect. Front Microbiol. 8:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popowska M. 2004. Analysis of the peptidoglycan hydrolases of Listeria monocytogenes: multiple enzymes with multiple functions. Pol J Microbiol. 53:29–34. [PubMed] [Google Scholar]
- Popowska M, Kusio M, Szymańska P, Markiewicz Z.. 2009. Inactivation of the wall-associated de-N-acetylase (PgdA) of Listeria monocytogenes results in greater susceptibility of the cells to induced autolysis. J Microbiol Biotechnol. 19:932–945. [DOI] [PubMed] [Google Scholar]
- Rai M, Yadav A, Gade A.. 2009. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 27:76–83. [DOI] [PubMed] [Google Scholar]
- Rice KC, Bayles KW.. 2008. Molecular control of bacterial death and lysis. Microbiol Mol Biol Bev. 72:85–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahverdi AR, Fakhimi A, Shahverdi HR, Minaian SM.. 2007. Synthesis and effect of silver nanoparticles on the antibacterial activity against Staphylococcus aureus and Escherichia coli. Nanomedicine 3: 168–171. [DOI] [PubMed] [Google Scholar]
- Singh M, Singh S, Prasada S, Gambhir IS.. 2008. Nanotechnology in medicine and antibacterial effect of silver nanoparticles. Digest J Nanomat Biostruct. 3:115–122. [Google Scholar]
- Smith JL, McColgan C, Marmer BS.. 1991. Growth temperature and the action of lysozyme on Listeria monocytogenes. J Food Sci. 56:1101–1102. [Google Scholar]
- Stapsford KE, Tyner KM, Dair BJ, Deschamps JR, Medintz IL.. 2011. Analyzing nanomaterial bioconjugates: a review of current and emerging purification and characterization techniques. Anal Chem. 83:4453–4488. [DOI] [PubMed] [Google Scholar]
- Tamayo LA, Zapata PA, Vejar ND, Azócar MI, Gulppi MA, Zhou X, Thompson GE, Rabagliati FM, Páez MA.. 2014. Release of silver and copper nanoparticles from polyethylene nanocomposites and their penetration into Listeria monocytogenes. Mater Sci Eng C Mater Biol Appl. 40:24–31. [DOI] [PubMed] [Google Scholar]
- Vázquez-Bolland JA, Kuhn M, Berche P, Chakraborthy T, Dominguez-Bernal G, Goebel W, González-Zorn B, Wehland J, Kreft J.. 2001. Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev. 14:584–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolska KI, Grudniak AM, Kamiński K, Markowska K.. 2015. The potential of metal nanoparticles for inhibition of bacterial biofilms In: Rai M, Kon K, editors. Nanotechnology in Diagnosis, Treatment and Prophylaxis of Infectious Diseases. Amsterdam (Netherlands): AP Elsevier; p. 119–132. [Google Scholar]


