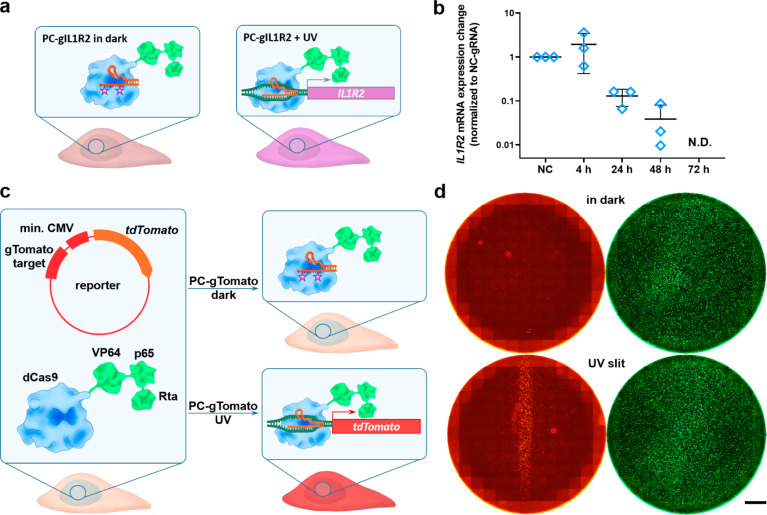
Figure 3.
Spatiotemporal control of gene expression using PC-gRNA. (a) Schematic representation of the experimental setup for the IL1R2 transcription activation. (b) Light-mediated expression of silent IL1R2 gene in HEK293FT cells. HEK-dCas9-VPR cells were transfected with the NC- or PC-gIL1R2 and were either kept in the dark or exposed to the UV light at 4–72 h post-transfection. The IL1R2 mRNA expression levels were determined 24 h postirradiation and were normalized to the NC-gIL1R2 treated samples (NC). The GAPDH mRNA expression level served as a reference. N.D.–not detected. (N = 3, mean ± SD). (c) Schematic representation of the experimental setup for visualization of transcription activation. HEK-dCas9-VPR cells were cotransfected with PC-gTomato and a reporter plasmid encoding tdTomato under minimal CMV promoter and containing the gRNA binding site. The uncaging of the PC-gTomato with the UV light leads to its binding to the complementary sequence in the reporter plasmid and enhances the tdTomato expression. (d) HEK-dCas9-VPR cells cotransfected with PC-gTomato, the tdTomato reporter plasmid, and a GFP plasmid as a transfection control, irradiated through a photomask. Transfection in a 24-well plate, tdTomato (red), GFP (green). Scale bar 2 mm.