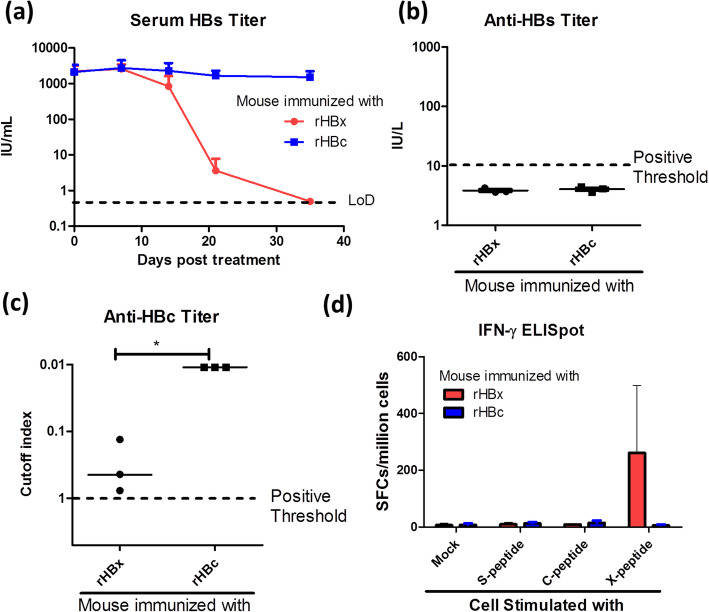
Fig. 4.
Comparison of the immunogenicity of HBV core antigen and that of HBV X protein. a-c HBV carrier mice (N = 3) received vaccination with 3.125 μg of rHBc or rHBx supplemented with 0.625 μg of CpG-ODN on days 0, 7 and 14. Mouse serum was sampled on days 0, 7, 14, 21 and 35. (a) Serum HBs titers were determined. b Serum anti-HBs antibody titer on day 35. c Serum anti-HBc antibody titer on day 35. Lower values represent higher antibody production because the anti-HBc assay is a competition assay. d HBV carrier mice (N = 3 ~ 4) received vaccination with 6.25 μg of rHBc or rHBx supplemented with 1.25 μg of CpG-ODN on days 0, 7, 14 and 22. Mice were boosted once with the same dose 7 days before splenocyte isolation. Splenocytes were separately stimulated with HBx-, HBs-, or HBc-derived 15-mer overlapping peptide pool and subjected to an IFN-γ ELISpot assay. The result was normalized to the spot counts per million cells. LoD, limit of detection; SFCs, spot-forming cells