Figure 2.
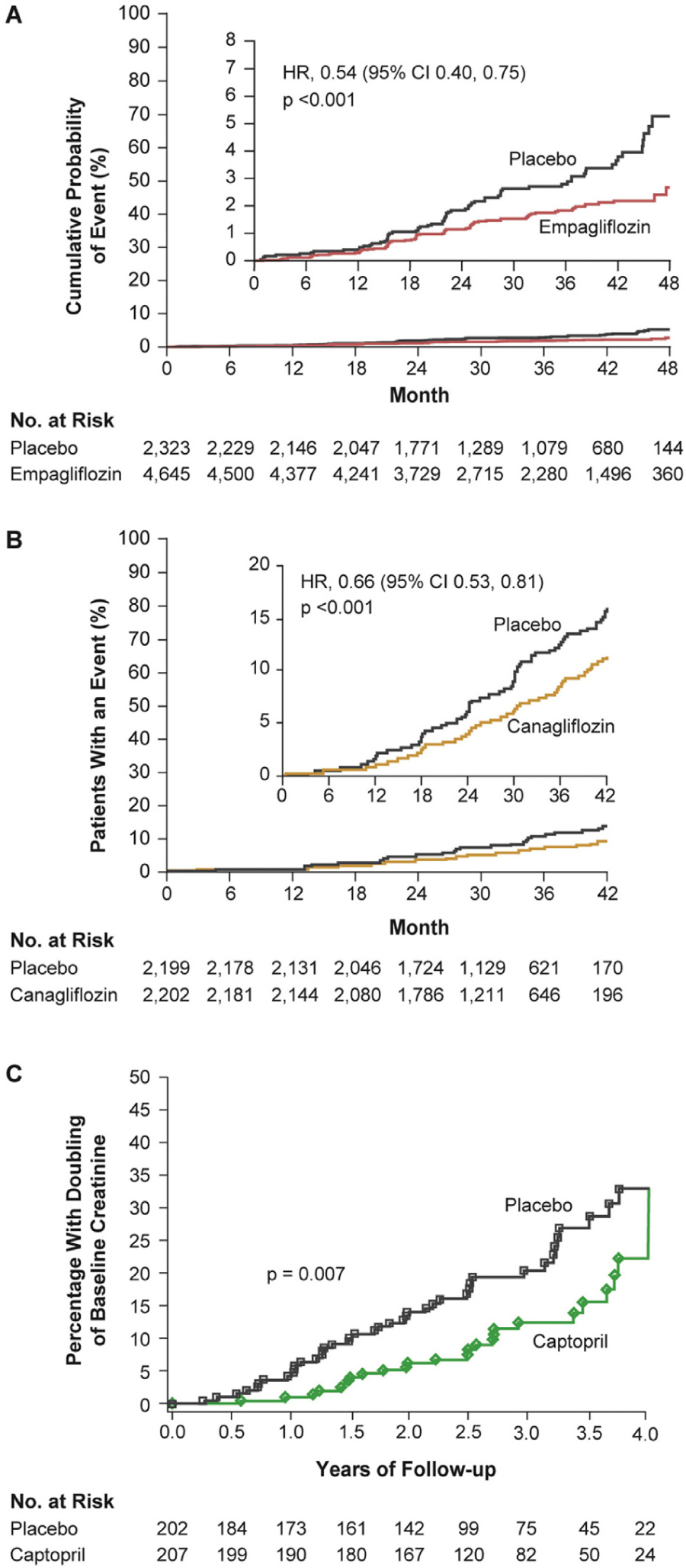
Renal outcomes in SGLT2 inhibitor trials of (A) empagliflozin (EMPA-REG OUTCOME)24 and (B) canagliflozin (CREDENCE)27 compared with (C) an earlier RAAS blocker (captopril) trial.28 (A) Renal-specific composite outcome of doubling of SCr level, initiation of renal replacement therapy, or death from renal disease in the EMPA-REG OUTCOME trial.24 From New England Journal of Medicine, Wanner C, et al., Empagliflozin and progression of kidney disease in type 2 diabetes, 375, 323–334. Copyright © 2016 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society. (B) Renal-specific composite outcome of ESRD, doubling of SCr level, or renal death in the CREDENCE trial.27 From New England Journal of Medicine, Perkovic V, et al. for the CREDENCE Trial Investigators, Canagliflozin and renal outcomes in type 2 diabetes and nephropathy, 380, 2295–2306. Copyright © 2019 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society. The placebo group was already receiving an ACEI or ARB and their rate of poor outcomes is remarkably similar to those who received captopril in the 1993 trial (~15% at 3.5 years28). (C) Renal end point of doubling of SCr level to ≥2.0 mg/dL in the 1993 captopril trial.28 From New England Journal of Medicine, Lewis EJ, et al., The effect of angiotensin-convertingenzyme inhibition on diabetic nephropathy, 329, 1456–1462, Copyright © 1993 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society. ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; RAAS = renin-angiotensin-aldosterone system; SCr = serum creatinine; SGLT2 = sodium-glucose co-transporter 2.
