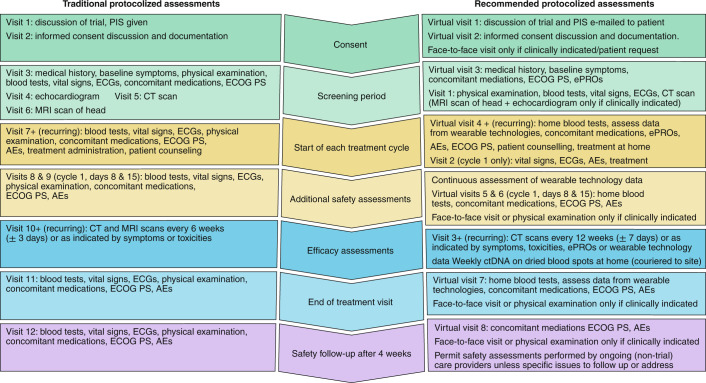
Fig. 2. Comparison of traditional and adapted clinical trial pipelines.
Side-by-side comparison of a traditional interventional cancer clinical trial assessment schedule with implications for patients, and the suggested protocol adaptations that might result in clinical cancer research that is more patient-centered, resilient and efficient. PIS, participant information sheet; ECG, electrocardiogram; ECOG PS, Eastern Cooperative Oncology Group Performance Status; CT, computed tomography; AEs, adverse events; MRI, magnetic resonance imaging.