Figure 7.1.
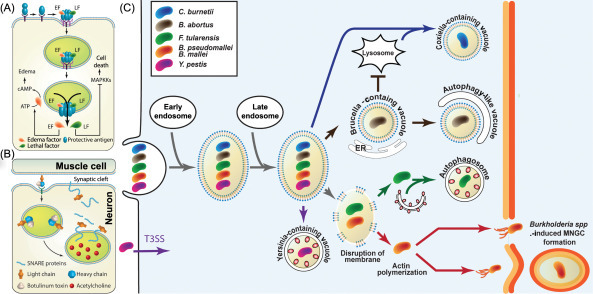
Mechanism of action of how bacterial pathogens invade, spread, and ultimately kill the mammalian host cell.
(A) Bacillus anthracis, a spore forming Gram-positive bacterium secretes the three proteins—PA, LF, and EF. These proteins form a pore-forming heterocomplex that undergoes receptor-mediated endocytosis. The acidic environment in the endosomes causing a conformational change in the PA protein thereby resulting in the translocation of the LF and EF into the cytosol of the cell. LF is a Zn-dependent metalloprotease that is known to cleave several members of the mitogen-activated protein kinase kinase family, thereby preventing interaction with and phosphorylation of downstream MAPK and ultimately resulting in disruption of host signaling pathways. EF is a calmodulin-dependent adenylate cyclase that modulates host response by producing increased levels of cyclic adenosine monophosphate (cAMP) and causing severe edema in infected host [11]. (B) BoNTs are secreted by the sporulating and anaerobic Gram-positive bacteria of the genus Clostridium. BoNTs are produced as inactive single-chain polypeptides (150 kDa) that are cleaved by proteases to form the pharmacologically active toxin consisting of the LC and HC that are linked by disulfide bridges. The HC component binds to the receptors on the neurons and mediates toxin insertion. Inside the neurons the LC that is a Zn-dependent metalloprotease cleaves the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins, thereby inhibiting the release of acetylcholine neurotransmitter into neuromuscular junctions and leading to neuroparalysis associated with botulism. (C) Intracellular bacterial pathogens share a number of mechanisms to enter, replicate, and disseminate; however, the repertoire of virulence factors that are unique to each pathogen dictate their intracellular niches. C. burnetii is unique in its ability to adapt the lysosome to create an ideal acidified vacuole for bacterial replication, called the Coxiella-containing vacuole. Brucella abortus is unique in its ability to acquire ER-derived membrane to create the Brucella-containing vacuole, where it can replicate. During late stages of infection Brucella spp. can convert vacuoles into autophagic vacuoles that facilitate bacterial egress and subsequent infections. Francisella tularensis can escape the vacuole and gain access to the cytosol of the cell where it can replicate to high numbers and late during infection in murine cells some cytosolic bacteria are found in autophagosomes and this population of surviving bacteria could be responsible for one mechanism of dissemination. Burkholderia pseudomallei and Burkholderia mallei also escape the phagosome and gain access to the cytosol where they replicate and spread from cell to cell using actin tails, resulting in the formation of MNGCs. Yersinia pestis is mainly an extracellular pathogen and secretes effectors using its T3SS; however, a few bacteria traffic intracellularly and reside within a Yersinia-containing vacuole that acquires autophagy markers, such as LC3. BoNT, botulinum neurotoxin; EF, edema factor; HC, heavy chain; LC, light chain; LF, lethal factor; MNGC, multinucleated giant cell; PA, protective antigen; T3SS, type 3 secretion system.
