Fig. 6. Observed intracellular conformations of AT1R.
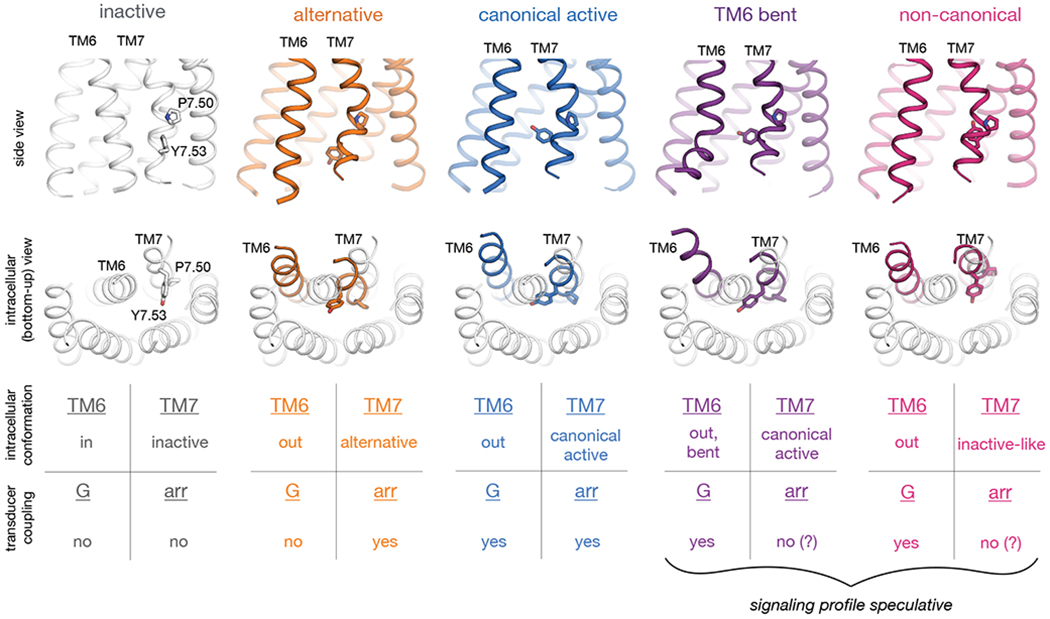
Simulations indicate that AT1R adopts at least five distinct conformations that differ substantially in the intracellular positions of TM6 and TM7 and may have distinct cellular signaling profiles. In the inactive conformation (gray; illustrated by crystal structure (24)), TM6 occludes the transducer-binding pocket, hindering coupling to either G proteins or arrestins. The remaining four conformations all exhibit more outward positions of TM6 but differ substantially in the conformation of TM7, as illustrated by representative frames from our AT1R simulations. In the middle row, we show TMs 6 and 7 of these four conformations (colors) overlaid on all TMs of the inactive structure (gray). Our results suggest that in the alternative conformation (orange), the intracellular surface hinders coupling to G proteins but allows for arrestin coupling, whereas the canonical active conformation (blue) couples to both G proteins and arrestins. Two other conformations that AT1R adopts in simulation—the TM6-bent and non-canonical conformations, in purple and pink, respectively—have been observed in G-protein-bound structures of other GPCRs but might hinder arrestin coupling by increasing the volume of the transducer-binding pocket, preventing the arrestin finger loop from packing tightly against this pocket.
