Abstract
Background and Aims:
Post-thoracotomy pain can be severe and disabling. The aim of this study was to examine the efficacy of intercostal nerve block when used as adjunct to thoracic epidural analgesia in patients undergoing posterolateral thoracotomy.
Methods:
This was a parallel-group randomised patient and assessor-blinded study carried out at a tertiary-referral cancer center. We included 60 adult patients undergoing elective lung resection under general anaesthesia with thoracic epidural analgesia. In addition, the intervention arm received single-shot intercostal blocks with 10 ml of 0.25% bupivacaine at the level of and two levels above and below the thoracotomy. We assessed post-operative pain scores at 2 to 4 hours and 18 to 24 hours after surgery, peri-operative fentanyl requirement, percentage of patients who needed fentanyl PCA and maximum volume achieved on bedside spirometry 18 to 24 hours after surgery. Groups were compared using the unpaired t-test for continuous data and the chi square test for categorical data at a 5% level of significance.
Results:
2 to 4 hours post-operatively, mean pain scores at rest were 3.0 in both groups (difference 0.04, 95% CI -1.1 to + 1.1) and on coughing were 4.6 (ICB group) and 4.9 (C group) (difference 0.32, 95% CI -1.0 to + 1.6). There were no differences between the groups for any of the other outcomes.
Conclusion:
Addition of intercostal block to epidural analgesia does not confer any benefit in terms of post-operative pain, fentanyl requirements or volume achieved on spirometry.
Keywords: Analgesia, epidural, intercostal nerve, thoracotomy
INTRODUCTION
Thoracic epidural analgesia (TEA) is recommended for post-thoracotomy analgesia because of its numerous benefits.[1] In addition to providing optimum relief of acute post-operative pain, the perioperative use of TEA has also been postulated to decrease the incidence of chronic post-thoracotomy pain.[2] However, epidural catheter placement can have failure rates as high as 30%.[3] Systemic opioids such as morphine and fentanyl are commonly used as rescue analgesics in such patients; however, these drugs have side effects like sedation, respiratory depression and nausea/vomiting.
While guidelines recommend intercostal block (ICB) as an alternative to TEA for the management of thoracotomy pain, it has also been suggested that a combination of TEA with ICB would be the ideal approach.[1,4] Only one previous study has evaluated the role of ICB as an adjunct to TEA for the management of post-thoracotomy pain.[4] We hypothesised that the addition of a multi-level intercostal block with local anaesthetic at the end of the surgery, under direct vision by the surgeon, would supplement thoracic epidural analgesia and reduce post-operative pain and the need for opioid rescue analgesic in patients undergoing thoracotomy.
The primary objective of the study was to compare pain scores 2 to 4 hours after surgery between 2 groups of patients undergoing standard posterolateral thoracotomy, who had a thoracic epidural catheter in situ, who had either received or not received additional one-time multi-segment intercostal block.
METHODS
This was a randomised parallel-group trial with patient and assessor blinding carried out in a high-volume thoracic surgical unit at a tertiary cancer centre. The Institutional Ethics Committee approved the study (approval number IEC/0815/1527/001 dated 18th August 2015) and it was registered prospectively with the Clinical Trials Registry of India (CTRI/2015/10/006283). All procedures performed in the study followed the ethical guidelines of the Declaration of Helsinki. Voluntary written informed consent was obtained from all participants and their parents when required. We included patients more than 15 years old, who were ASA physical status I, II and III, and undergoing standard open posterolateral thoracotomy for lung resections. Patients who refused consent, who had a contra-indication to epidural, contra-indication to any of the study drugs, additional surgical incisions during the same sitting (except mediastinoscopy), previous thoracotomy/thoracoscopy, previous radiation therapy to the thorax and chronic pre-operative pain (defined as use of pain medications continuously for more than 1 week in the 4 weeks preceding surgery) were excluded.
As part of standard pre-operative preparation, a physiotherapist evaluated all patients planned for lung resection and taught them breathing exercises and incentive spirometry. Patients performed these exercises for at least two weeks prior to surgery. A research physiotherapist visited the patient on the day before surgery and recorded the maximum volume achieved on bedside spirometry (Leventon Spiro-Ball®). Prior to induction of general anaesthesia, patients had an epidural catheter placed at the mid-thoracic level. A test dose consisting of 3 ml of 1.5% lignocaine and 15 micrograms of adrenaline was injected through the catheter to rule out intravascular or intrathecal placement. Once a negative response to test dose was established, patients received general anaesthesia with intravenous (IV) fentanyl 2 μg per kg per kilogram, IV propofol titrated to loss of verbal response and IV vecuronium 0.1 mg per kg. The choice of airway device (single lumen endotracheal tube with or without bronchial blocker or double lumen endobronchial tube) was left to the discretion of the anaesthesiologist. Prior to surgical incision, 6 to 8 ml of 0.1% bupivacaine with 2 μg per ml of fentanyl was injected through the epidural catheter. If the patient was haemodynamically unstable after induction of anaesthesia (defined as a fall in systolic blood pressure by more than 30% from baseline or less than 90 mm Hg), treatment was given in the form of intravenous fluids (boluses of 5 ml per kg) and mephentermine (6 mg bolus) and the initial epidural dose was delayed until the patient was haemodynamically stable. Subsequent to the bolus, patients received an epidural infusion of 0.1% bupivacaine with 2 μg per ml of fentanyl at 6 to 8 ml per hour.
The surgical procedure was performed as per standard protocol. The operating room anaesthesiologist could give additional boluses of fentanyl, 0.5 μg per kg if heart rate and blood pressure exceeded baseline by more than 30%.
At the end of the surgery, before closure of thoracotomy, patients were randomised to one of the study groups: Intercostal block (ICB) group or control group (C). Randomisation was carried out using a computer-generated list by a member of the centrally-located Clinical Research Secretariat, who was not involved in any aspect of patient care. Serially numbered opaque sequential envelopes were used to maintain allocation concealment. Patients received one of the following interventions:
ICB group: Patients received ICB with 0.25% bupivacaine at the level of the thoracotomy and at two levels above and below the level of thoracotomy, with 2 ml of solution per level using a 22-gauge needle. The ICB was given by the operating surgeon under direct vision, between the dorsal end of the parietal pleura incision and the costovertebral junction.
C group: Patients did not receive any ICB.
The operative notes and anaesthesia records did not carry details of the randomisation arm or intervention. All patients received intravenous diclofenac 1 mg per kg, to a maximum of 75 mg before extubation. After tracheal extubation, patients were shifted to the recovery room. Epidural analgesia was continued as an infusion of 0.1% bupivacaine with 2 μg per ml of fentanyl at 6 to 8 ml per hour. The recovery room anaesthesiologist (who was also blinded to the intervention) managed post-operative pain in a standardised manner as per departmental protocols. Patients with a functioning epidural catheter received continuous epidural infusion via an elastomeric pump using a mixture of bupivacaine 0.1% and fentanyl 2 mcg per ml at 6 to 8 ml per hour. Post-operatively, all patients received intravenous diclofenac 1 mg per kg (to a maximum of 75 mg) 8 hourly. If pain scores remained above 4, 30 minutes after receiving diclofenac, patients received intravenous Paracetamol 20 mg per kg (to a maximum of 1 gram) 8 hourly. Patients with a non-functioning epidural catheter and patients who had moderate to severe pain, defined as pain score more than 4 on a Numerical Rating Scale of 1 to 10, despite a functioning epidural catheter were considered for an intravenous fentanyl patient-controlled analgesia (PCA) pump (bolus 20 μg, lock-out period 15 min). Patients, recovery room personnel and acute pain services personnel were all blinded to the study arm. Mid-way during the study, after 34 patients had been randomised (18 on treatment arm and 16 on control arm), the acute pain services revised their pain management protocol and increased the concentration of fentanyl in the epidural elastomeric pump to 5 mcg/ml. Since this was a randomised study and affected both arms equally, it was decided to continue with the protocol.
A blinded research nurse assessed pain at two time-points: First, between two and four hours after surgery and second, between 18 to 24 hours after surgery. We used these time points to capture the efficacy of the block (at 2 to 4 hours) and the pain after the effect of the block wore off (18 to 24 hours). Patients described their pain using a numerical rating scale where 0 represented no pain and 10 represented the worst imaginable pain. In between these time points, patients were assessed and treated by the Acute Pain Services of the hospital as part of routine care. Any interventions performed by the Acute Pain Services were captured in our database. We also calculated the mean requirement of fentanyl (μg per kg) post-operatively up to 24 hours after surgery, percentage of patients in each group who needed fentanyl PCA pump post-operatively and maximum volume achieved on bedside spirometry 18 to 24 hours after surgery, expressed as a percentage of baseline maximum spirometry volume.
Data was entered into a statistical software package for analysis (SPSS for Windows, Version 20.0). Analysis was performed on an intention-to-treat basis. Comparisons between groups were carried out using the unpaired t test for continuous data and the Chi-square test for categorical data. 'p' values less than 0.05 were considered significant for all comparisons and no adjustment was made for multiple comparisons. Based on observational data from 20 patients who underwent thoracotomy with epidural analgesia, the mean pain score 2 to 4 hours after surgery was found to be 6 (on NRS from 1 to 10) with a standard deviation of 1.5. To detect a change by 2 points with 80% power at 5% level of significance, we would need 30 patients in each arm.
RESULTS
Between January 2016 and November 2017, 60 patients were accrued on this study (30 on each arm). Out of 83 patients who met the eligibility criteria, 11 refused consent, 6 had failed epidural insertions, and 6 could not be randomised as a member of the research team was not present. Figure 1 shows the CONSORT flowchart of patients. As shown in Table 1, the groups were well matched with respect to baseline characteristics. Table 2 shows the data for the primary and secondary outcomes. There was no difference between the two groups for any of the study outcomes. Importantly, postoperative pain scores at various time points were similar in the two groups as was the rescue analgesia requirements and ability of patients to perform chest physiotherapy and spirometry. Four patients in the control arm (13%) and 5 patients in the treatment arm (17%) needed PCA. This difference was not statistically significant. The reasons for the PCA are mentioned in Table 3. Two patients in the control arm and four patients in the treatment arm had their epidural infusions stopped transiently during surgery in view of hypotension. Of these, in one patient in the treatment arm, there was intra-operative vascular injury with major bleeding. He did not receive the intra-operative dose of diclofenac and inadvertently, was not given the intercostal block. One patient in the treatment arm developed a post-operative cerebrovascular event with dense hemiplegia. He was subsequently intubated and mechanically ventilated and could not be assessed for study outcomes. 2 patients on the control arm were mechanically ventilated in the post-operative period due to poor respiratory attempts and could not be assessed for pain at 2 to 4 hours. 2 patients in the control arm and one in the treatment arm missed their spirometry assessment at 18 to 24 hours.
Figure 1.
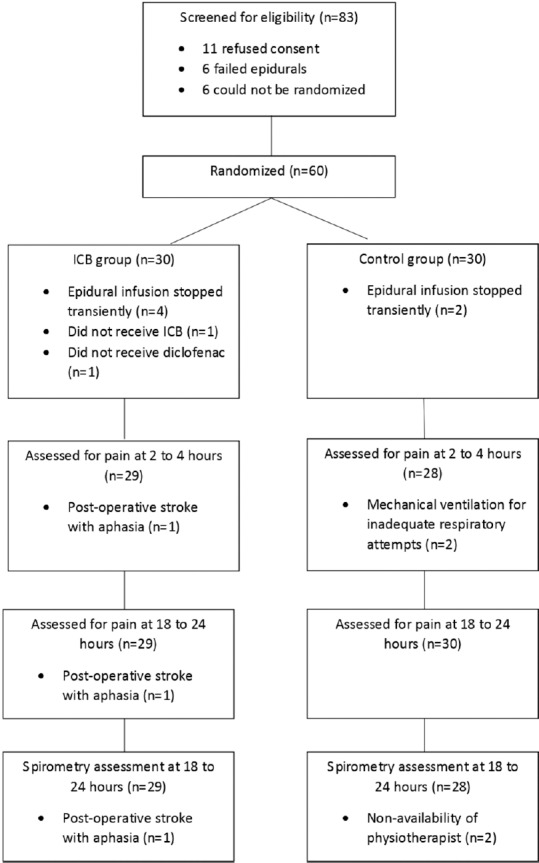
CONSORT flowchart of patients
Table 1.
Baseline characteristics
| Variable | ICB group (n=30) | C group (n=30) |
|---|---|---|
| Age (in years) | 47.1 (16.2) | 45.6 (14.2) |
| Gender | ||
| Male | 21 | 25 |
| Female | 9 | 5 |
| Weight (in kilogram) | 61.6 (12.2) | 60.1 (13.0) |
| Height in centimetres | 166.1 (19.3) | 164.0 (8.8) |
| Baseline volume achieved on spirometry (in ml) | 1976.8 (547.5) | 1909.2 (387.6) |
| Type of surgery | ||
| Lobectomy | 10 (33.3%) | 22 (73.3%) |
| Pneumonectomy | 10 (33.3%) | 5 (16.7%) |
| Others | 10 (33.3%) | 3 (10%) |
| Number of patients with ribs broken intra-operatively | 11 (37%) | 14 (47%) |
| Intra-operative fentanyl requirement (µg/kg) | 3.45 (1.2) | 3.74 (1.2) |
Data is expressed as mean (with standard deviation in brackets) for continuous data and actual numbers (with percentages in brackets) for categorical data
Table 2.
Primary and secondary outcomes
| Outcome | ICB group | C group | P | Difference between means with 95% confidence intervals |
|---|---|---|---|---|
| Pain at 2 to 4 hours post-operatively* [n=29(ICB), 28(C)] | ||||
| At rest | 3.0 (2.0) | 3.0 (2.2) | 0.95 | 0.04 (-1.1 to+1.1) |
| On coughing | 4.6 (2.2) | 4.9 (2.7) | 0.62 | 0.32 (-1.0 to+1.6) |
| Pain at 18 to 24 hours post-operatively* [n=29(ICB), 30(C)] | ||||
| At rest | 2.1 (1.6) | 2.4 (1.6) | 0.53 | 0.26 (-0.6 to+1.1) |
| On coughing | 4.0 (1.7) | 4.4 (2.2) | 0.35 | 0.49 (-0.6 to+1.5) |
| Maximum volume achieved on spirometry (absolute value in ml) [n=29(ICB), 28(C)] | 1148 (350) | 1062 (352) | 0.37 | 94 (-275 to+104) |
| Maximum volume achieved on spirometry (percentage of baseline) | 0.6 (0.2) | 0.6 (0.2) | 0.50 | -0.04 (-0.14 to+0.05) |
| Postoperative fentanyl requirements (mcg/kg) | 4.4 (3.1) | 5.0 (2.1) | 0.39 | 0.6 (-1.1 to+2.3) |
Data is expressed as mean (with standard deviation in brackets) *Pain scores are reported on a Numerical Rating Scale of 1 to 10
Table 3.
Reasons for PCA
| ICB group (n=29) | C group (n=30) | |
|---|---|---|
| Non-functioning epidural | 1 | 0 |
| Post-operative hypotension necessitating discontinuation of epidural | 2 | 0 |
| Inadequate analgesia despite functioning epidural | 3 | 4 |
DISCUSSION
In this randomised trial, we found that addition of intercostal block to epidural analgesia did not offer any advantage in terms of pain scores, need for adjuvant analgesia or performance on spirometry. Post-thoracotomy pain is one of the most severe types of postoperative pain and may impact postoperative pulmonary and cardiac complications.[2] The management of post-thoracotomy pain has been the subject of several studies and guidelines suggest that thoracic epidural analgesia (TEA) should be the gold standard.[1] However, TEA may be ineffective in some cases. This may be due to incorrect placement, secondary migration of catheter, sub-optimal doses of epidural drugs (volume or concentration) or patchy or unilateral effect of drugs.[3] Patients undergoing major surgery may be haemodynamically unstable in the immediate post-operative period, necessitating the discontinuation of epidural local anaesthetic. It is therefore possible, that a certain proportion of patients may have significant pain in the immediate post-operative period, despite having an epidural catheter in situ.
Alternatives to TEA include paravertebral, intercostal, inter-pleural and subarachnoid blocks.[1,2] Of these, ICB given by the surgeon is appealing since it is given under direct vision and has minimal side effects. The objective of our study was to look at the efficacy of ICB as an adjunct to TEA for post-thoracotomy pain. We found no difference in immediate or delayed postoperative pain experienced by patients, requirement for additional analgesics or impact on pulmonary function.
Previous studies have compared TEA with ICB for the treatment of post-thoracotomy pain.[5,6,7,8,9,10] Out of six studies comparing single shot intercostal techniques with TEA, 5 studies found equivalent analgesia whereas one study found that ICB was inferior. In many of these studies, patients received intravenous opioids to supplement the ICB. With growing concern about the disadvantages of opioids for postoperative pain in patients undergoing cancer surgery, the utility of this technique remains debatable.[11,12] Only 2 studies have compared TEA with infusions through intercostal catheters, the results of which are inconclusive.[13,14] A systematic review by Detterbeck looked at the efficacy of methods of intercostal nerve blockade for pain relief after thoracotomy and concluded that while extra-pleural infusion is at least as effective as an epidural and significantly better than narcotics alone, other techniques of intercostal blockade do not offer an advantage over narcotics alone.[15] It is known that intercostal catheter placement may be technically difficult and therefore, the results of this review may not be generalisable.
Pertunnen showed that segmental spread of analgesia was comparable between a single-shot 4-segment ICB and continuous paravertebral and epidural blocks up to 20 hours after injection.[9] A subsequent study by Wurnig corroborated this by showing that pain scores were better with ICB on postop day 1 and were better in the TEA group from postop day 2 suggesting that ICB provides adequate blockade of intercostal nerves for the first 24 hours after surgery.[7] They suggested that a combination of these two techniques would be the ideal method for post-thoracotomy analgesia. These studies formed the basis for our hypothesis.
Only one previous study by Takamori and colleagues has looked at ICB as an adjunct to TEA.[4] They measured pain twice a day for 5 days, using 2 different scales and found significant differences in pain scores. This study did not use narcotics, which could explain the higher pain scores than seen in other studies. They also used non-steroidal analgesics only on demand. It is now accepted that multi-modal or balanced analgesia enhances recovery after surgery and that the use of NSAIDs, where not contra-indicated, may contribute to decreasing the inflammatory response.[16] The use of NSAIDs also helps to treat pain unrelated to the thoracotomy e.g. shoulder pain from positioning. Unlike Takamori's study, in our study, patients received non-steroidal analgesics round-the-clock.
As a post-hoc analysis, we attempted to study if, in patients with ineffective epidurals, the intercostal block offered any advantage. However, we had inadequate patients in this sub-group to allow meaningful analysis. It is possible that in a different group of patients (e.g., esophagectomies), where fluid shifts and haemodynamic changes are more prevalent, and the chances of having an epidural infusion terminated are higher, the use of ICB as an adjunct to TEA would be beneficial.
The strength of our study is that we minimised bias at multiple levels by ensuring randomisation, allocation concealment, patient and assessor blinding and standardisation of perioperative pain management techniques. One of the limitations of our study is that we focused on immediate outcomes and did not study chronic pain, pulmonary complications or hospital outcomes. However, with no difference in acute pain or pulmonary functions, it is unlikely that long-term outcomes would be impacted. Another drawback is the small sample size of our study; however, our sample size was based on the anticipated difference in pain scores between the two groups, and any smaller difference (which would have been the only way we could have justified a larger sample size) would not have been clinically meaningful.
The main disadvantage of a single-shot intercostal block with plain bupivacaine is the limited duration of action. Studies have looked at single-shot intercostal block with liposomal bupivacaine with promising results.[17,18,19] However, they are all non-randomised small studies and the cost of liposomal bupivacaine needs to be balanced with the benefits.
In conclusion, our study failed to show any benefit of a single-shot, multi-level intercostal block when used as an adjunct with thoracic epidural analgesia for the treatment of post-thoracotomy pain.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient and parent consent forms. In the form, the patient(s) and/or parents has/have given his/her/their consent/assent for his/her/their child's images and other clinical information to be reported in the journal. The patients and/or parents understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
This study was funded by a research grant from the Department of Atomic Energy Clinical Trials Centre, Tata Memorial Centre, Mumbai, India
REFERENCES
- 1.Prospect – Procedure specific post-operative pain management. [Internet] [Last cited on 2018 Dec 17]. Available from: http://www.postoppain.org/frameset.htm .
- 2.De Cosmo G, Aceto P, Gualtieri E, Congedo E. Analgesia in thoracic surgery: Review. Minerva Anestesiol. 2009;75:393–400. [PubMed] [Google Scholar]
- 3.Hermanides J, Hollmann MW, Stevens MF, Lirk P. Failed epidural: Causes and management. Br J Anaesth. 2012;109:144–54. doi: 10.1093/bja/aes214. [DOI] [PubMed] [Google Scholar]
- 4.Takamori S, Yoshida S, Hayashi A, Matsuo T, Mitsuoka M, Shirouzu K. Intraoperative intercostal nerve blockade for postthoracotomy pain. Ann Thorac Surg. 2002;74:338–41. doi: 10.1016/s0003-4975(02)03710-4. [DOI] [PubMed] [Google Scholar]
- 5.Concha M, Dagnino J, Cariaga M, Aguilera J, Aparicio R, Guerrero M. Analgesia after thoracotomy: Epidural fentanyl/bupivacaine compared with intercostal nerve block plus intravenous morphine. J Cardiothorac Vasc Anesth. 2004;18:322–6. doi: 10.1053/j.jvca.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Luketich JD, Land SR, Sullivan EA, Alvelo-Rivera M, Ward J, Buenaventura PO, et al. Thoracic epidural versus intercostal nerve catheter plus patient-controlled analgesia: A randomized study. Ann Thorac Surg. 2005;79:1845. doi: 10.1016/j.athoracsur.2004.10.055. [DOI] [PubMed] [Google Scholar]
- 7.Wurnig PN, Lackner H, Teiner C, Hollaus PH, Pospisil M, Fohsl-Grande B, et al. Is intercostal block for pain management in thoracic surgery more successful than epidural anaesthesia? Eur J Cardiothorac Surg. 2002;21:1115–9. doi: 10.1016/s1010-7940(02)00117-3. [DOI] [PubMed] [Google Scholar]
- 8.Bachmann-Mennenga B, Biscoping J, Kuhn DF, Schürg R, Ryan B, Erkens U, et al. Intercostal nerve block, interpleural analgesia, thoracic epidural block or systemic opioid application for pain relief after thoracotomy? Eur J Cardiothorac Surg. 1993;7:12–8. doi: 10.1016/1010-7940(93)90141-w. [DOI] [PubMed] [Google Scholar]
- 9.Perttunen K, Nilsson E, Heinonen J, Hirvisalo EL, Salo JA, Kalso E. Extradural, paravertebral and intercostal nerve blocks for post-thoracotomy pain. Br J Anaesth. 1995;75:541–7. doi: 10.1093/bja/75.5.541. [DOI] [PubMed] [Google Scholar]
- 10.Meierhenrich R, Hock D, Kühn S, Baltes E, Muehling B, Muche R, et al. Analgesia and pulmonary function after lung surgery: Is a single intercostal nerve block plus patient-controlled intravenous morphine as effective as patient-controlled epidural anaesthesia? A randomized non-inferiority clinical trial. Br J Anaesth. 2011;106:580–9. doi: 10.1093/bja/aeq418. [DOI] [PubMed] [Google Scholar]
- 11.Oh TK, Jeon JH, Lee JM, Kim MS, Kim JH, Lim H, et al. Association of high-dose postoperative opioids with recurrence risk in esophageal squamous cell carcinoma: Reinterpreting ERAS protocols for long-term oncologic surgery outcomes. Dis Esophagus. 2017;30:1–8. doi: 10.1093/dote/dox074. [DOI] [PubMed] [Google Scholar]
- 12.Maher DP, Wong W, White PF, McKenna R, Jr, Rosner H, Shamloo B, et al. Association of increased postoperative opioid administration with non-small-cell lung cancer recurrence: A retrospective analysis. Br J Anaesth. 2014;113(Suppl 1):i88–94. doi: 10.1093/bja/aeu192. [DOI] [PubMed] [Google Scholar]
- 13.Kaiser AM, Zollinger A, De Lorenzi D, Largiadèr F, Weder W. Prospective, randomized comparison of extrapleural versus epidural analgesia for postthoracotomy pain. Ann Thorac Surg. 1998;66:367–72. doi: 10.1016/s0003-4975(98)00448-2. [DOI] [PubMed] [Google Scholar]
- 14.Debreceni G, Molnár Z, Szélig L, Molnár TF. Continuous epidural or intercostal analgesia following thoracotomy: A prospective randomized double-blind clinical trial. Acta Anaesthesiol Scand. 2003;47:1091–5. doi: 10.1034/j.1399-6576.2003.00208.x. [DOI] [PubMed] [Google Scholar]
- 15.Detterbeck FC. Efficacy of methods of intercostal nerve blockade for pain relief after thoracotomy. Ann Thorac Surg. 2005;80:1550–9. doi: 10.1016/j.athoracsur.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 16.Wick EC, Grant MC, Wu CL. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: A review. JAMA Surg. 2017;152:691–7. doi: 10.1001/jamasurg.2017.0898. [DOI] [PubMed] [Google Scholar]
- 17.Khalil KG, Boutrous ML, Irani AD, Miller CC, 3rd, Pawelek TR, Estrera AL, et al. Operative intercostal nerve blocks with long-acting bupivacaine liposome for pain control after thoracotomy. Ann Thorac Surg. 2015;100:2013–8. doi: 10.1016/j.athoracsur.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 18.Mehran RJ, Walsh GL, Zalpour A, Cata JP, Correa AM, Antonoff MB, et al. Intercostal nerve blocks with liposomal bupivacaine: Demonstration of safety, and potential benefits. Semin Thorac Cardiovasc Surg. 2017;29:531–7. doi: 10.1053/j.semtcvs.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Rice DC, Cata JP, Mena GE, Rodriguez-Restrepo A, Correa AM, Mehran RJ. Posterior intercostal nerve block with liposomal bupivacaine: An alternative to thoracic epidural analgesia. Ann Thorac Surg. 2015;99:1953–60. doi: 10.1016/j.athoracsur.2015.02.074. [DOI] [PubMed] [Google Scholar]


