Figure 5 |. RapGEF2 or membrane-associated guanylate kinase 2 (MAGI2) knockdown in podocytes similarly diminish Rap1 activation and Rap1-mediated downstream signaling.
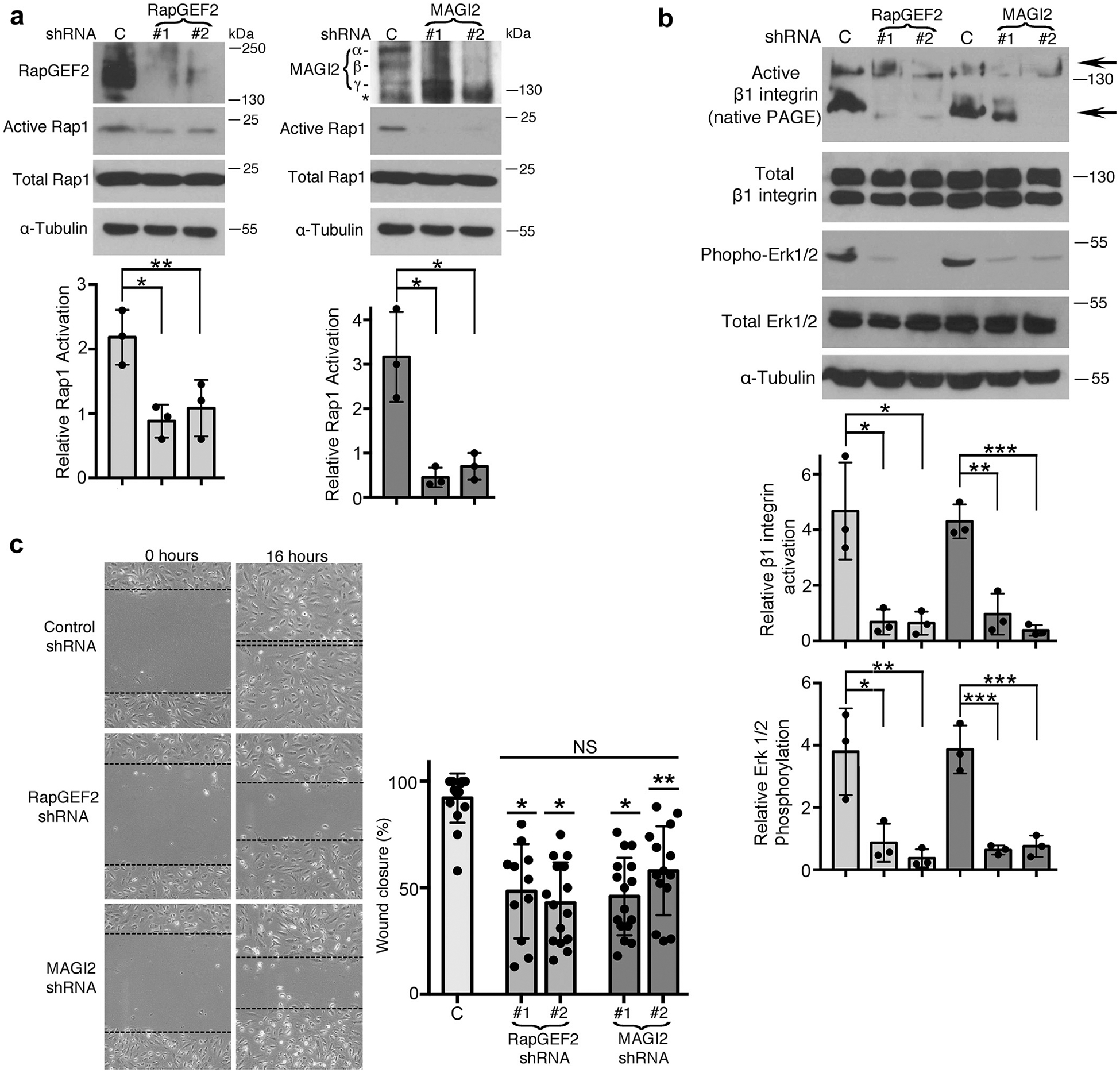
(a) Human cultured podocytes transduced with short hairpin (sh)RNA expression plasmid targeting RapGEF2 or MAGI2 mRNA demonstrated diminished RapGEF2 or MAGI2 protein expression, respectively, compared with podocytes expressing a scrambled shRNA. Two distinct shRNA plasmids were used for both RapGEF2 and for MAGI2, each with a unique mRNA target sequence. Levels of Rap1 activation induced by calcium switch were dramatically reduced in all 4 knockdown cell lines compared with control cells. Ratio of active to total Rap1 was calculated for 3 individual experiments and then normalized (for control cells vs. RapGEF2 #1 and #2, *P < 0.03, **P < 0.04; for control cells vs. MAGI2 #1 and #2, *P < 0.01 for both). α, β, and γ indicate major MAGI2 splice isoforms. The asterisk represents a nonspecific band. (b) Western blotting of podocytes transduced as indicated was performed after calcium switch. Levels of β1 integrin activation and Erk1/2 phosphorylation (both major Rap1 downstream signals) weresubstantially diminished. Densitometric ratios of active to total β1 integrin were calculated for 3 individual experiments and then normalized. *P < 0.02, **P < 0.01, ***P < 0.001. Quantification combined the intensities of the 2 active bands for β1 integrin (shown by arrows; the upper 130 kDa band represents its fully mature glycosylated form, and the lower 110 kDa band represents its precursor form) and compared it with the combined intensities of the mature and immature forms of total β1 integrin. Densitometric quantificationfor phospho to total Erk1/2 is also shown. *P < 0.03, **P < 0.02, ***P < 0.01. (c) RapGEF2 and MAGI2 knockdown cells migratedmore slowly than control cells. The percent wound closure was quantified at fixed locations along the scratch. *P < 0.0001, **P < 0.002 (each vs. control subjects). NS, not significant; PAGE, polyacrylamide gel electrophoresis.
