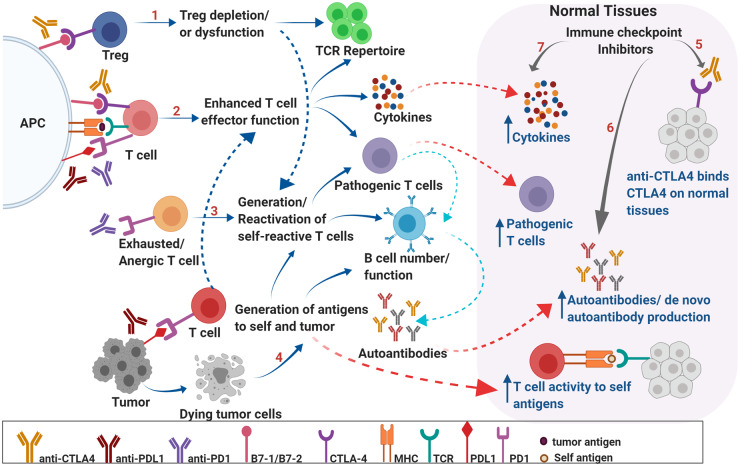
Fig. 1.
Mechanisms underlying irAEs. Schematic representation of potential mechanisms underlying irAEs associated with ICI therapy. CTLA-4 expression on regulatory T cells is critical in maintaining peripheral tolerance by preventing activation of self-reactive T cells. CTLA-4 blockade may result in depletion of Tregs, may alter Treg function, and may modulate T-cell repertoire, resulting in autoreactive T cells that can, in turn, affect B-cell function and increased autoantibody production (A). PD-1- and CTLA-4-mediated negative regulation of T cells is critical in maintaining self-tolerance via suppressing costimulation. PD-1 and CTLA-4 blockade results in enhanced effector function of T cells and may lead to generation of pathogenic T cells, overproduction of cytokines, alteration of B cell numbers/function and increased production of autoantibodies leading to inflammation and autoimmunity (B). PD-1 blockade may result in reactivation of exhausted/anergic T cells resulting in pathogenic/self-reactive T cells (C). Epitope spreading can lead to breakdown of tolerance. Tumor cell death results in production of self and tumor antigens that are ingested by APCs, which migrate to lymph nodes and prime T cells. These self-reactive T cells can reenter normal tissues, recognize self-antigens, and result in increased cytokine production and autoantibodies leading to breakdown of tolerance and tissue destruction (D). ICI therapy may directly result in breakdown of tolerance in organs via binding to CTLA-4 expressed on normal tissues (E); production of de novo autoantibodies and/or increased levels of preexisting autoantibodies (F); and increased levels of pro inflammatory cytokines locally, leading to infiltration of pathogenic immune cells (G). These factors can generate a local inflammatory milieu resulting in organ damage. APC, antigen-presenting cell; MHC, major histocompatibility complex; Treg, regulatory T cell; TCR, T-cell receptor.