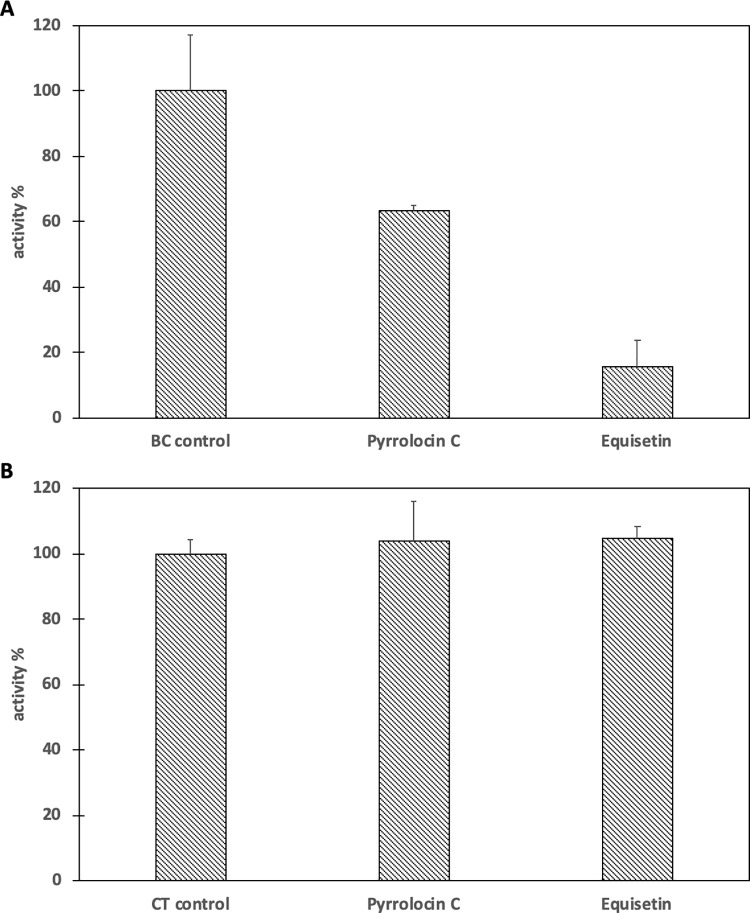
Fig 8. Inhibition of the biotin carboxylase (BC) and carboxyltransferase (CT) components of acetyl-CoA carboxylase by Pyrrolocin C and Equisetin.
The concentration of each inhibitor was 0.1 mM. A. Percent activity of biotin carboxylase in the presence of Pyrrolocin C or Equisetin. The initial velocity of biotin carboxylase in the absence of inhibitor was measured and set to 100%. The substrates biotin (40 mM) and ATP (0.2 mM) were held constant at subsaturating levels. B. Percent activity of carboxyltransferase in the presence of Pyrrolocin C or Equisetin. The initial velocity of carboxyltransferase in the absence of inhibitor was measured and set to 100%. The substrates biocytin (6 mM) and malonyl-CoA (0.04 mM) were held constant at subsaturating levels. All assays were performed in triplicate ± S.D.