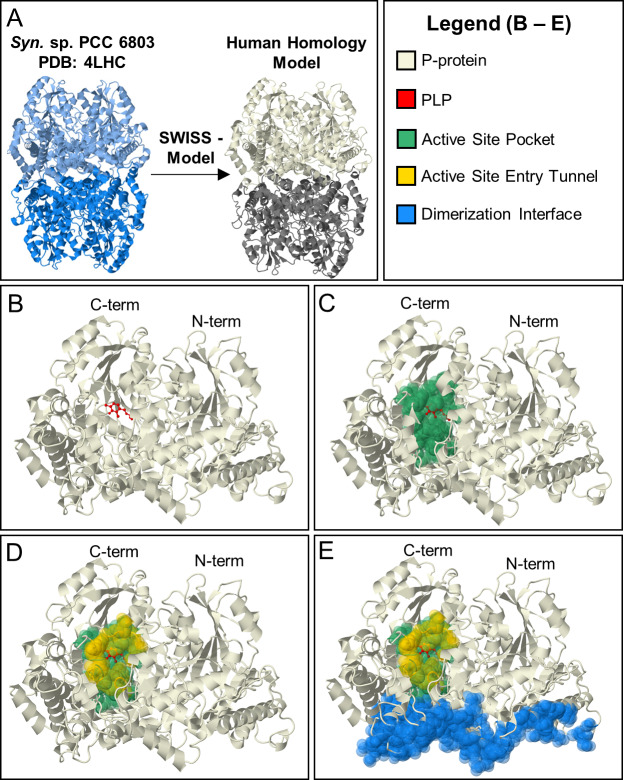
Fig 2. Human homology model of P-protein.
[A]. A crystal structure of Synechocystis sp. PCC 6833 GLDC holoenzyme (PDB = 4LHC) (LHS) was the template used in SWISS-Model to generate the human homology model (RHS). Based on homology, human GLDC is predicted to form an α2 homodimer. α-subunit shown in beige. α’ shown in gray. [B–E] Evolutionary derived, functional regions of human GLDC model with. [B] cofactor PLP (shown in red); [C] active site pocket (green) defined as residues within 5 Angstroms of PLP containing active site lysine (K754), [D] a tunnel (shown in yellow) that opens at the surface when PLP is bound making the active site pocket accessible to lipoylated H-protein; [E] the dimerization interface of GLDC (blue).