Fig. 1. Fabrication and charge transport characterization of graphene M-2D-vdWHs.
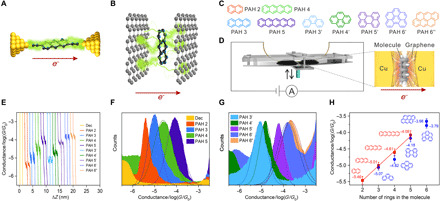
Illustrations of in-plane (A) and cross-plane (B) charge transport. Green trajectories are indicative of electron scattering paths. (C) Chemical structures of the polycyclic aromatic hydrocarbons (PAHs) that sandwiched between two graphene electrodes. In particular, naphthalene (PAH 2), anthracene (PAH 3), tetracene (PAH 4), and pentacene (PAH 5) are linear PAHs, while phenanthrene (PAH 3′), pyrene (PAH 4′), perylene (PAH 5′), benzoperylene (PAH 6′), and anthanthrene (PAH 6″) are nonlinear PAHs. (D) Schematic of the cross-plane break junction (XPBJ) technique and the device structure of the studied graphene M-2D-vdWHs. (E) Examples of conductance versus displacement traces measured with PAHs and traces measured without the PAHs (yellow, Dec). The traces are shifted horizontally for clarity. 1D conductance histograms generated from ~1000 traces for graphene M-2D-vdWH of the linear PAHs (F) and the nonlinear PAHs (G). (H) The single-molecule conductance of each graphene M-2D-vdWH, plotted as a function of the number of benzene rings of the sandwiched molecule. The error bar is determined from the chip-to-chip variation of three independent experiments.
