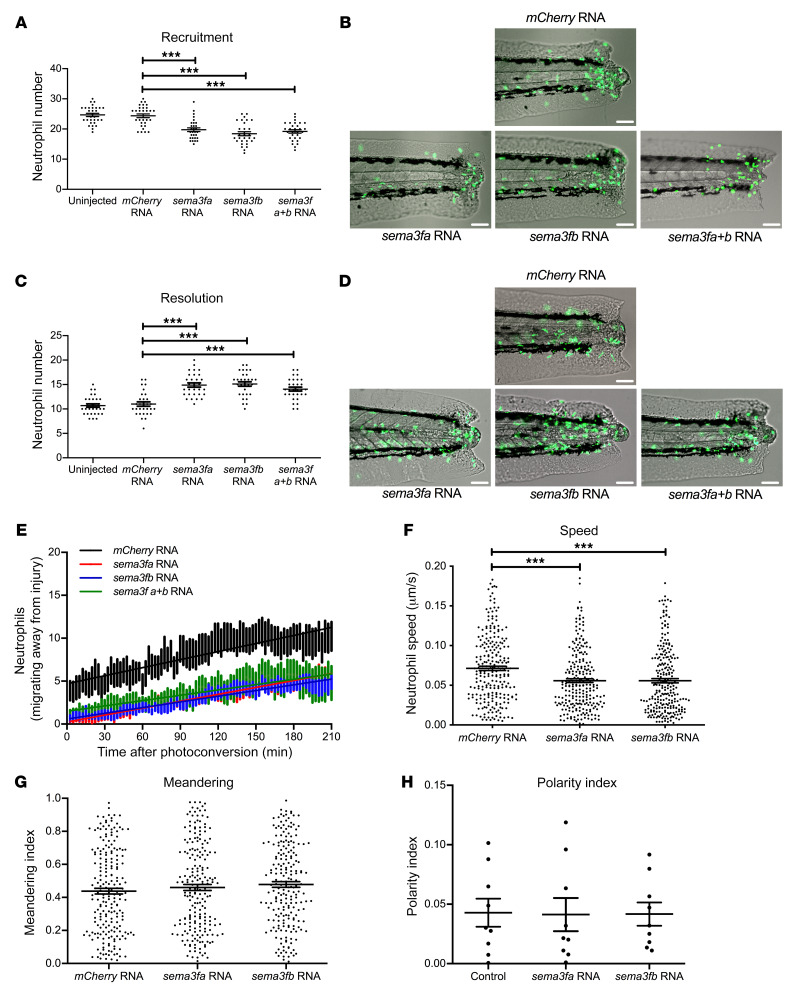
Figure 5. Overexpression of sema3f in a zebrafish model of inflammation delays neutrophil recruitment and resolution of the inflammatory response.
(A–D) sema3fa or sema3fb RNA (50 ng/μL) was injected into 1-cell-stage zebrafish mpx:GFP embryos, with 50 ng/μL mCherry RNA used as a negative control. Tail fin transection was performed at 2 dpf, and neutrophils counted at 6 and 24 hpi. (A) Neutrophil counts at the 6 hpi time point with (B) overlaid fluorescence and bright-field photomicrographs (recruitment). (C) Neutrophil counts at the 24 hpi time point with (D) overlaid fluorescence and bright-field photomicrographs (resolution). Scale bars: 60 μm (B and D). Data are mean ± SEM with individual data points from 3 independent experiments (n = 30). (E) sema3fa and/or sema3fb RNA (50 ng/μL) were injected into 1-cell-stage zebrafish mpx:kaede embryos, and tail fin transection was performed at 2 dpf. Neutrophils recruited to the wound at 6 hpi were photoconverted, and red fluorescent neutrophils were tracked for 3.5 hours. Data are mean ± SEM from 3 independent experiments (n = 9). (F and G) sema3fa or sema3fb RNA (50 ng/μL) was injected into 1-cell-stage zebrafish mpx:GFP embryos, with 50 ng/μL mCherry RNA used for control. Tail fin transection was performed at 2 dpf. Neutrophil movement was tracked over 1 hour by time-lapse microscopy during the recruitment phase of inflammation (1–2 hpi) and speed of neutrophil migration (F) and meandering index (displacement/path length) (G) were determined. Data are mean ± SEM from 3 independent experiments (each point represents a single neutrophil) (n = 15). (H) sema3fa or sema3fb RNA (50 ng/μL) was injected into 1-cell-stage Tg(lyz:PHAkt-EGFP) embryos with noninjected controls, tail fin transection was performed at 2 dpf, and polarity indices were calculated for neutrophils recruited to the tail region. Data are mean ± SEM from a single experiment (n = 8). Statistical analysis was by 1-way ANOVA and Bonferroni’s post hoc test. ***P < 0.001.